
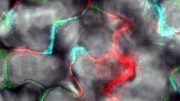
Timelapse of plastic degradation. A new enzyme variant can break down plastics that typically take centuries to degrade in just a matter of hours to days.
A new enzyme variant can break down environment-throttling plastics that typically take centuries to degrade in just a matter of hours to days. It was created by chemical engineers and scientists at The University of Texas at Austin
This discovery, published on April 27, 2022, in the journal Nature, could help solve one of the world’s biggest environmental problems: what to do with the billions of tons of plastic waste piling up in landfills and polluting our natural lands and water. The enzyme has the potential to supercharge recycling on a large scale that would enable major industries to reduce their environmental impact by recovering and reusing plastics at the molecular level.
“The possibilities are endless across industries to leverage this leading-edge recycling process,” said Hal Alper, professor in the McKetta Department of Chemical Engineering at UT Austin. “Beyond the obvious waste management industry, this also provides corporations from every sector the opportunity to take a lead in recycling their products. Through these more sustainable enzyme approaches, we can begin to envision a true circular plastics economy.”
Includes timelapse of plastic degradation over 48-hour period. Credit: The University of Texas at Austin / Cockrell School of Engineering
The project focuses on polyethylene terephthalate (PET), a significant polymer found in most consumer packaging, including cookie containers, soda bottles, fruit and salad packaging, and certain fibers and textiles. It makes up 12% of all global waste.
The enzyme was able to complete a “circular process” of breaking down the plastic into smaller parts (depolymerization) and then chemically putting it back together (repolymerization). In some cases, these plastics can be fully broken down to monomers in as little as 24 hours.
PET (polyethylene terephthalate) is the most common thermoplastic polymer resin of the polyester family and is used in fibers for clothing, containers for liquids and foods, and thermoforming for manufacturing.
Researchers at the Cockrell School of Engineering and College of Natural Sciences used a machine learning model to generate novel mutations to a natural enzyme called PETase that allows bacteria to degrade PET plastics. The model predicts which mutations in these enzymes would accomplish the goal of quickly depolymerizing post-consumer waste plastic at low temperatures.
Through this process, which included studying 51 different post-consumer plastic containers, five different polyester fibers and fabrics, and water bottles all made from PET, the researchers proved the effectiveness of the enzyme, which they are calling FAST-PETase (functional, active, stable, and tolerant PETase).
“This work really demonstrates the power of bringing together different disciplines, from synthetic biology to chemical engineering to artificial intelligence,” said Andrew Ellington, professor in the Center for Systems and Synthetic Biology whose team led the development of the machine learning model.
Recycling is the most obvious way to cut down on plastic waste. But globally, less than 10% of all plastic has been recycled. The most common method for disposing of plastic, besides throwing it in a landfill, is to burn it, which is costly, energy-intensive, and spews noxious gas into the air. Other alternative industrial processes include very energy-intensive processes of glycolysis, pyrolysis, and/or methanolysis.
Biological solutions take much less energy. Research on enzymes for plastic recycling has advanced during the past 15 years. However, until now, no one had been able to figure out how to make enzymes that could operate efficiently at low temperatures to make them both portable and affordable at large industrial scale. FAST-PETase can perform the process at less than 50 degrees Celsius (122 degrees Fahrenheit).
Up next, the team plans to work on scaling up enzyme production to prepare for industrial and environmental application. The researchers have filed a patent application for the technology and are eying several different uses. Cleaning up landfills and greening high waste-producing industries are the most obvious. But another key potential use is environmental remediation. The team is looking at a number of ways to get the enzymes out into the field to clean up polluted sites.
“When considering environmental cleanup applications, you need an enzyme that can work in the environment at ambient temperature. This requirement is where our tech has a huge advantage in the future,” Alper said.
Reference: “Machine learning-aided engineering of hydrolases for PET depolymerization” by Hongyuan Lu, Daniel J. Diaz, Natalie J. Czarnecki, Congzhi Zhu, Wantae Kim, Raghav Shroff, Daniel J. Acosta, Bradley R. Alexander, Hannah O. Cole, Yan Zhang, Nathaniel A. Lynd, Andrew D. Ellington and Hal S. Alper, 27 April 2022, Nature.
DOI: 10.1038/s41586-022-04599-z
Alper, Ellington, associate professor of chemical engineering Nathaniel Lynd and Hongyuan Lu, a postdoctoral researcher in Alper’s lab, led the research. Danny Diaz, a member of Ellington’s lab, created the machine learning model. Other team members include from chemical engineering: Natalie Czarnecki, Congzhi Zhu and Wantae Kim; and from molecular biosciences: Daniel Acosta, Brad Alexander, Yan Jessie Zhang and Raghav Shroff. The work was funded by ExxonMobil’s research and engineering division as part of an ongoing research agreement with UT Austin.







What happens if the mutated enzyme is picked up and incorporated into the DNA of wild bacteria? What would happen if we could no longer use PET and similar plastics for industrial and consumer applications? The world as we know it could change in a matter of weeks as all the potable water in warehouses leaks out, and millions of people die of thirst before glass bottles can be produced to replace them. And, consider the extra energy required to produce and ship heavy glass bottles around the world to provide people with water who don’t have reliable potable water locally.
These myopic researchers should keep in mind the immutable Murphy’s Law: If something CAN go wrong it will. It is probably best to design for a system outside the ambient conditions so that it can be kept contained in the industrial plants.
A great idea for my next Sci Fi disaster book.
Clyde Spencer, I think you should be writing science fiction. Please explain how an enzyme – a 3d protein entity – can be read and reverse translated and transcribed into DNA? It you can give me even one instance of this happening, i will be happy to continue this discussion with you.
Is this what you were asking for?
https://study.com/learn/lesson/reverse-transcriptase-overview-function-structure.html
I’m still waiting for an answer, Thanki.
Are the depolymerization products at least as safe as the PET itself? It’s not uncommon for polymers to have monomers and plasticizers that are quite toxic.