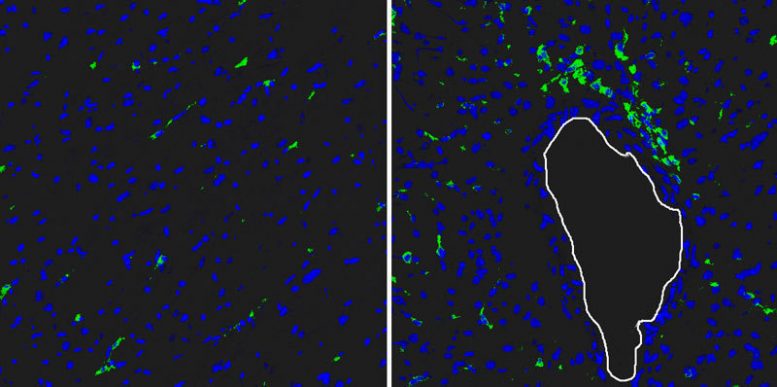
Reg3beta attracts white blood cells to the infarct area: After injection of Reg3beta, many more macrophages (stained green) migrate into the infarct tissue (right) than without injection (left). The nuclei of various cells are stained blue. The white contour shows the outline of a blood vessel. Credit: MPI f. Heart and Lung Research
A new study from the Max Planck Institute details how Reg3beta controls the wound-healing process in the myocardium by attracting immune cells to the infarct tissue.
In the wake of a myocardial infarction, parts of the myocardium die and are replaced by scar tissue. The formation and stability of scar tissue are key to the survival of patients following an acute myocardial infarction. Scientists at the Max Planck Institute for Heart and Lung Research in Bad Nauheim have now discovered a gene, known as Reg3beta, which plays an important role in healing damaged tissue in the heart.
Acute blood flow impairment of the myocardium, as occurs during a cardiac infarction, has dramatic consequences for the surrounding heart region: if doctors are unable to dissolve the obstruction in the blood vessel quickly, muscle cells affected by the lack of adequate blood flow will die. This has fatal consequences for the patient, because in humans, once myocardial tissue is lost, it can no longer be regenerated. The pumping performance of the heart remains impaired for life.
If poor perfusion persists, numerous remodeling processes occur in the damaged part of the myocardium, resulting in replacement of the dead muscle tissue by scar tissue. Experts refer to this process as wound healing, even if the heart is no longer intact afterwards. “Without this remodeling, the heart would sooner or later tear. An optimum wound-healing and scar-forming process is therefore important for the mid- and long-term prognosis of infarction patients,” says Jochen Pöling, doctor and scientist at the Max Planck Institute for Heart and Lung Research.
The researchers in Bad Nauheim used a mouse model to investigate the underlying healing process in damaged hearts. In doing so, they discovered important information on the role of the immune system. “Just a few hours after an infarction, white blood cells migrate into the damaged muscle. The first to arrive are granulocytes, which primarily remove dead myocardial cells. Subsequently, cells of the immune system known as macrophages migrate into the area. Our data show that macrophages are particularly important for a coordinated and optimum healing process,” says Holger Lörchner, lead author of the study.
In their study, the researchers investigated hundreds of proteins that are expressed by surviving myocardial cells. In the process, they discovered Reg3beta. In subsequent experiments, it became clear that Reg3beta acts as an attractant of macrophages, specifically promoting their migration into the damaged myocardium. “In mice lacking the Reg3beta gene, far fewer immune cells migrate into the infarction area,” says Lörchner. The result is a disturbed wound-healing process, which has fatal consequences for the animal’s survival. “In comparison to controls with normal Reg3beta activity, we observed cardiac rupture much more frequently in animals without Reg3beta. The heart literally rips.”
The Bad Nauheim-based researchers conclude from their study that Reg3beta plays a key role in the regulation of wound healing. “Because of the reduced migration of macrophages, we observed a disturbed wound-healing process. Thus, the connective tissue produced in mice without Reg3beta was significantly poorer than in control animals. In addition, far fewer new blood vessels formed in the infarction region. Ultimately, this leads to an unstable scar,” says Pöling.
In another experiment, the researchers were able to compensate for the negative effects caused by a lack of Reg3beta. Using a complicated method, they artificially injected Reg3beta directly into the scar tissue. Subsequently, the wound-healing process was essentially normal. “We therefore plan to investigate whether Reg3beta is a potential candidate for use in acute myocardial infarction to improve wound-healing processes in damaged myocardium,” says Thomas Braun, Director at the Max Planck Institute. It is hoped that Reg3beta therapy will improve the stability of the heart and reduce potential consequences such as heart failure.
Reference: “Myocardial healing requires Reg3β-dependent accumulation of macrophages in the ischemic heart” by Holger Lörchner, Jochen Pöling, Praveen Gajawada, Yunlong Hou, Viktoria Polyakova, Sawa Kostin, Juan M Adrian-Segarra, Thomas Boettger, Astrid Wietelmann, Henning Warnecke, Manfred Richter, Thomas Kubin and Thomas Braun, 9 March 2015, Nature Medicine.
DOI: 10.1038/nm.3816








Be the first to comment on "Reg3beta Regulates Healing Process after Myocardial Infarction"