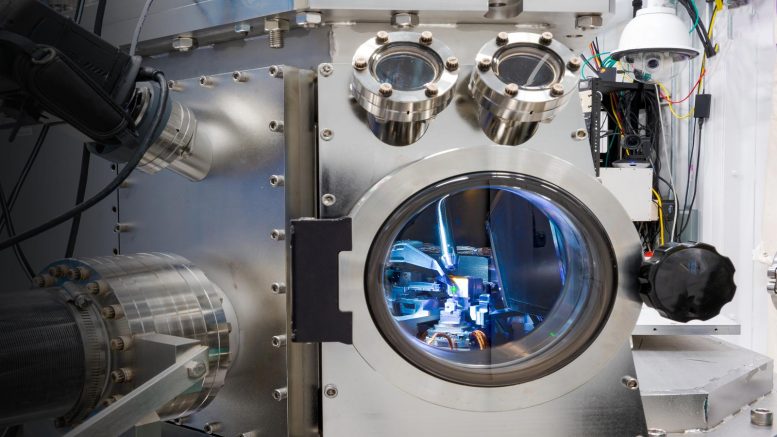
Looking into the hard X-ray nanoprobe synchrotron chamber while measuring a response of an individual cuprous oxide particle to the exposure of carbon dioxide, water, and light. Credit: Tijana Rajh / Argonne National Laboratory
Scientists devise new catalyst that uses light to turn carbon dioxide to fuel.
The concentration of carbon dioxide in our atmosphere is steadily increasing, and many scientists believe that it is causing impacts in our environment. Recently, scientists have sought ways to recapture some of the carbon in the atmosphere and potentially turn it into usable fuel — which would be a holy grail for sustainable energy production.
In a recent study from the U.S. Department of Energy’s (DOE) Argonne National Laboratory, scientists have used sunlight and a catalyst largely made of copper to transform carbon dioxide to methanol. A liquid fuel, methanol offers the potential for industry to find an additional source to meet America’s energy needs.
“Carbon dioxide is such a stable molecule and it results from the burning of basically everything, so the question is how do we fight nature and go from a really stable end product to something useful and energy rich.” — Argonne Distinguished Fellow Tijana Rajh
The study describes a photocatalyst made of cuprous oxide (Cu2O), a semiconductor that when exposed to light can produce electrons that become available to react with, or reduce, many compounds. After being excited, electrons leave a positive hole in the catalyst’s lower-energy valence band that, in turn, can oxidize water.
“This photocatalyst is particularly exciting because it has one of the most negative conduction bands that we’ve used, which means that the electrons have more potential energy available to do reactions,” said Argonne Distinguished Fellow Tijana Rajh, an author of the study.
Previous attempts to use photocatalysts, such as titanium dioxide, to reduce carbon dioxide tended to produce a whole mish-mash of various products, ranging from aldehydes to methane. The lack of selectivity of these reactions made it difficult to segregate a usable fuel stream, Rajh explained.
“Carbon dioxide is such a stable molecule and it results from the burning of basically everything, so the question is how do we fight nature and go from a really stable end product to something useful and energy-rich,” Rajh said.
The idea for transforming carbon dioxide into useful energy comes from the one place in nature where this happens regularly. “We had this idea of copying photosynthesis, which uses carbon dioxide to make food, so why couldn’t we use it to make fuel?” Rajh said. “It turns out to be a complex problem, because to make methanol, you need not just one electron but six.”
By switching from titanium dioxide to cuprous oxide, scientists developed a catalyst that not only had a more negative conduction band but that would also be dramatically more selective in terms of its products. This selectivity results not only from the chemistry of cuprous oxide but from the geometry of the catalyst itself.
“With nanoscience, we start having the ability to meddle with the surfaces to induce certain hotspots or change the surface structure, cause strain or certain surface sites to expose differently than they are in the bulk,” Rajh said.
Because of this “meddling,” Rajh and Argonne postdoctoral researcher Yimin Wu, now an assistant professor at the University of Waterloo, managed to create a catalyst with a bit of a split personality. The cuprous oxide microparticles they developed have different facets, much like a diamond has different facets. Many of the facets of the microparticle are inert, but one is very active in driving the reduction of carbon dioxide to methanol.
According to Rajh, the reason that this facet is so active lies in two unique aspects. First, the carbon dioxide molecule bonds to it in such a way that the structure of the molecule actually bends slightly, diminishing the amount of energy it takes to reduce. Second, water molecules are also absorbed very near to where the carbon dioxide molecules are absorbed.
“In order to make fuel, you not only need to have carbon dioxide to be reduced, you need to have water to be oxidized,” Rajh said. “Also, adsorption conformation in photocatalysis is extremely important — if you have one molecule of carbon dioxide absorbed in one way, it might be completely useless. But if it is in a bent structure, it lowers the energy to be reduced.”
Argonne scientists also used scanning fluorescence X-ray microscopy at Argonne’s Advanced Photon Source (APS) and transmission electron microscopy at the Center for Nanoscale Materials (CNM) to reveal the nature of the faceted cuprous oxide microparticles. The APS and CNM are both DOE Office of Science User Facilities.
A paper based on the study, “Facet-dependent active sites of a single Cu2O particle photocatalyst for CO2 reduction to methanol,” appeared in the November 4 online edition of Nature Energy. Other contributors to the study include Argonne’s Ian McNulty, Cong Liu, Kah Chun Lau, Paul Paulikas, Cheng-Jun Sun, Zhonghou Chai, Jeff Guest, Yang Ren, Vojislav Stamenkovic, Larry Curtiss and Yuzi Liu. Qi Liu of the City University of Hong Kong also contributed.
Reference: “Facet-dependent active sites of a single Cu2O particle photocatalyst for CO2 reduction to methanol” by Yimin A. Wu, Ian McNulty, Cong Liu, Kah Chun Lau, Qi Liu, Arvydas P. Paulikas, Cheng-Jun Sun, Zhonghou Cai, Jeffrey R. Guest, Yang Ren, Vojislav Stamenkovic, Larry A. Curtiss, Yuzi Liu and Tijana Rajh, 4 November 2019, Nature Energy.
DOI: 10.1038/s41560-019-0490-3
The work was funded by an Argonne Laboratory-Directed Research and Development grant and by the DOE’s Office of Science.





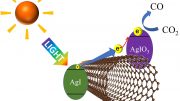


But surely regardless of any catalytic reaction, the energy required to turn CO2 into methanol requires at least the same amount of energy that is evolved when methanol is burned, which is 715 kJ mol-1. Where does this energy come from?
Excellent question. I always wonder about this when I hear about “new” means of creating useful energy to replace fossil fuels, the latest being the “blue energy” concept I have been reading about. Does the energy output exceed the total energy needed to create the means to extract/harness it? One of the famous Odom brother’s ecologists once questioned if nuclear energy was a net energy producer because ot the tremendous amounts of energy used to dispose of the radioactive waste products – (Umm a good question indeed!). While I certainly hope technology can find the means to replace fossil fuels in the near future, the net energy question always needs to be asked and answered.
I think we already know what the net energy return on investment is for most energy cycles, and by far nuclear has the highest return on investment and the lowest CO2 emmisions per unit of energy produced by far. We can’t allow for fossil fuel because the CO2 breaks the system and hasn’t and never can be carbon neutral. Solar and wind can be positive, but solar barely comes out positive when the intermitency is factored in since these both rely on fossil fuels for backup.
If you absolutely forbid CO2 outputs then solar and wind become untenable because we usually use 70% gas or coal and mix the other 30% from solar and wind. If you increase the solar wind mix you lose your grid, the gas backup is the CO2 achiles heel of renewables. It is ofcourse worse since the peaker gas power plants run at 40% best efficiency while the base load CCNG plant can hit 60% but those don’t want to be cut out by intermittents. In other words, wind plus peakers releases more CO2 than does a much more efficient CCNG plant. In theory a CCNG power plant that burns in pure O2 rather than air can even hit 80% efficiency but with a 10% tax to make the O2 inputs.
Now nuclear has some tiny CO2 emissions too, but that is many orders lower than fossil fuel, so a tiny percent of nuclear electric output could be set aside to pull CO2 from the oceans.
And the energy needed to pull CO2 from the oceans is about the same as the energy produced by the nat gas that released said CO2 decades before in the air.
In otherwords a nat gas power plant that pulls it’s own CO2 emmisions worth from the oceans is 100% taxed, a fools errand, no net energy left and this is what we propose to back up renewables. Worse you have only broken down methane into useless CO2 now in hand.
We know how to make cheap hydrogen from high temp nuclear heat with the S-I process, we know the energy cost of neutral CO2 from the oceans, the petro chemists could figure the entire cost of CO2 neutral synfuels, and it will be more than fossil fuels, but that may be fine for aviation fuels, but maybe not land fuels.