
Duke-NUS Medical School has developed a promising stem cell therapy for heart failure, where pluripotent stem cells grown in the lab can repair damaged tissue and improve heart function when transplanted into a damaged heart. This innovative procedure could be transformative for patients with heart failure, reducing the risk of complications like arrhythmias or tumors while promoting the regeneration of healthy heart tissue.
These stem cells have been shown to repair diseased cells, providing a promising remedy for individuals suffering from heart failure.
Scientists at Duke-NUS Medical School have developed a stem cell therapy for heart failure that has demonstrated potential in preclinical trials. The therapy involves transplanting these cells into a damaged heart, which can subsequently mend the injured tissue and enhance the heart function, as reported in their study recently published in the journal npj Regenerative Medicine.
Ischaemic heart disease, characterized by a reduction in blood supply to the heart, is currently the leading cause of death globally. This happens when the heart’s blood flow is obstructed, leading to the death of the heart muscle cells—a situation known as myocardial infarction, or more commonly referred to as a heart attack.
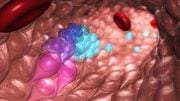
In this study, a unique new protocol was used where pluripotent, or immature, stem cells were cultivated in the laboratory to grow into heart muscle precursor cells, which can develop into various types of heart cells. This is done through cell differentiation, a process by which dividing cells gain specialized functions. During preclinical trials, the precursor cells were injected into the area of the heart damaged by myocardial infarction, where they were able to grow into new heart muscle cells, restoring damaged tissue and improving heart function.
“As early as four weeks after the injection, there was rapid engraftment, which means the body is accepting the transplanted stem cells. We also observed the growth of new heart tissue and an increase in functional development, suggesting that our protocol has the potential to be developed into an effective and safe means for cell therapy,” said Dr. Lynn Yap, an assistant professor at Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore. As the study’s first author, Dr. Yap led the research while she was an assistant professor with Duke-NUS’ Cardiovascular & Metabolic Disorders (CVMD) Programme.
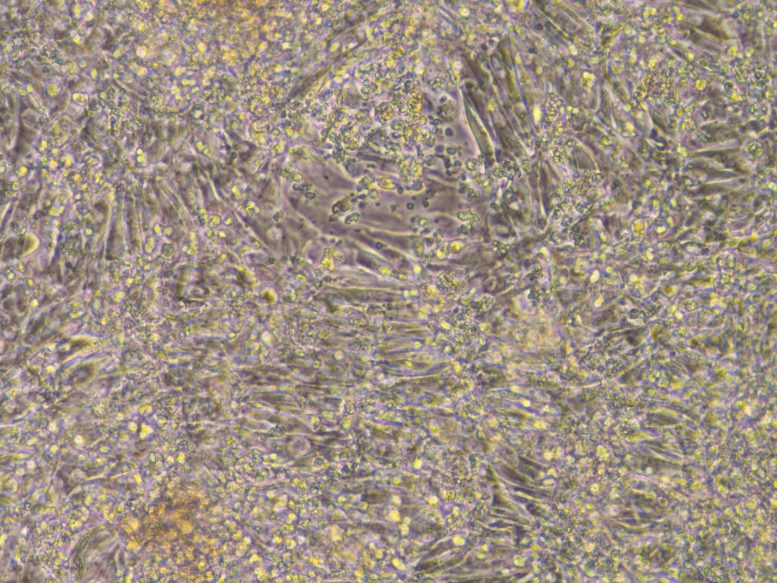
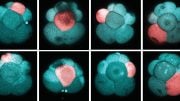
Human heart cells that are beating. Credit: Prof Karl Tryggvason & Dr. Lynn Yap
In studies conducted by other groups, the transplantation of heart muscle cells that were already beating brought about fatal side effects—namely, ventricular arrhythmia—abnormal heartbeats that can limit or stop the heart from supplying blood to the body.
The new procedure developed by Duke-NUS researchers involves transplanting non-beating heart cells into the damaged heart. After the transplantation, the cells expanded and acquired the rhythm of the rest of the heart. With this procedure, the incidence of arrhythmia was cut by half. Even when the condition was detected, most episodes were temporary and self-resolved in around 30 days. In addition, the transplanted cells did not trigger tumor formation—another common concern when it comes to stem cell therapies.
“Our technology brings us a step closer to offering a new treatment for heart failure patients, who would otherwise live with diseased hearts and have slim chances of recovery. It will also have a major impact in the field of regenerative cardiology, by offering a tried-and-tested protocol that can restore damaged heart muscles while reducing the risk of adverse side effects,” said Professor Karl Tryggvason from Duke-NUS’ CVMD Programme and the senior author of the study.
Prof Tryggvason, who is also the Tanoto Foundation Professor in Diabetes Research, is leading other studies to adapt this regenerative medicine method for patients with diabetes, macular degeneration in the eyes, and those needing skin grafts.
Underpinning all these studies is a controllable, stable, and reproducible method to make the right cells for transplantation using laminins—proteins that have a major role in the interactions of cells with their surrounding structures. Laminins exist in different forms depending on their environment and play a key role in directing the development of specific tissue cell types. In this study, stem cells were differentiated into heart muscle cells by growing them on the type of laminin abundantly found in the heart.
“To ensure patient safety, it is imperative that cell-based therapies show consistent efficacy and reproducible results. By extensive molecular and gene expression analyses, we demonstrated that our laminin-based protocol for generating functional cells to treat heart disease is highly reproducible,” said Associate Professor Enrico Petretto, co-author of the study and Director of the Centre for Computational Biology at Duke-NUS.
Reference: “Pluripotent stem cell-derived committed cardiac progenitors remuscularize damaged ischemic hearts and improve their function in pigs” by Lynn Yap, Li Yen Chong, Clarissa Tan, Swarnaseetha Adusumalli, Millie Seow, Jing Guo, Zuhua Cai, Sze Jie Loo, Eric Lim, Ru San Tan, Elina Grishina, Poh Loong Soong, Narayan Lath, Lei Ye, Enrico Petretto and Karl Tryggvason, 26 May 2023, npj Regenerative Medicine.
DOI: 10.1038/s41536-023-00302-6
The technology has been licensed to a Swedish biotech startup earlier this year to further promote the development of cell-based regenerative cardiology.


Be the first to comment on "Revolutionary Stem Cell Therapy Offers New Hope for Heart Failure Patients"