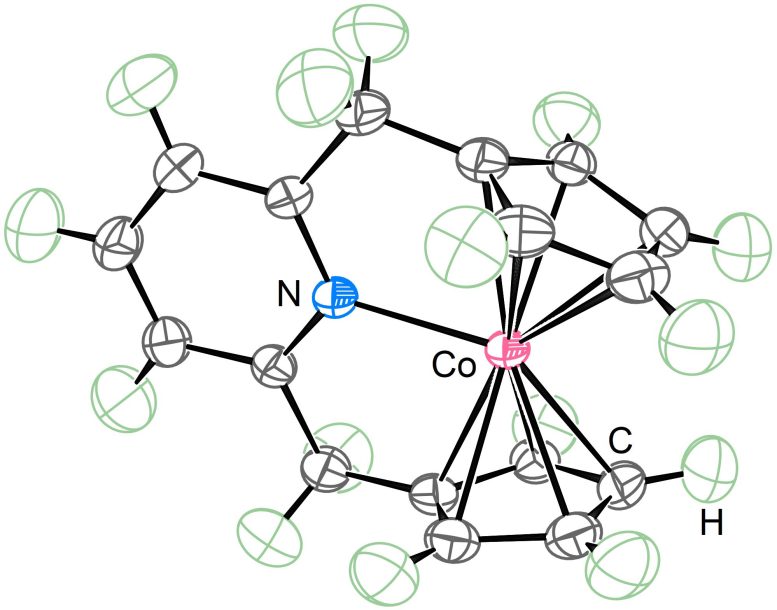
Crystal structure of the newly synthesized 21-electron metallocene compound showing the nitrogen (blue), cobalt (red), hydrogen (green), and carbon (grey) atoms. Credit: Takebayashi et al., 2023
The finding has potential to open new directions in medicine, catalysis, and energy.
Organometallic compounds, molecules made up of metal atoms and organic molecules, are often used to accelerate chemical reactions and have played a significant role in advancing the field of chemistry.
Metallocenes
Metallocenes, a type of organometallic compound, are known for their versatility and special “sandwich” structure. Their discovery was a significant contribution to the field of organometallic chemistry and led to the awarding of the Nobel Prize in Chemistry in 1973 to the scientists who discovered and explained their sandwich structure.
The versatility of metallocenes is due to their ability to “sandwich” many different elements to form a variety of compounds. They can be used in various applications, including the production of polymers, glucometers – used to measure the amount of glucose in the blood, perovskite solar cells, and as a catalyst, a substance that increases the rate of a chemical reaction without being consumed or changed by the reaction itself.
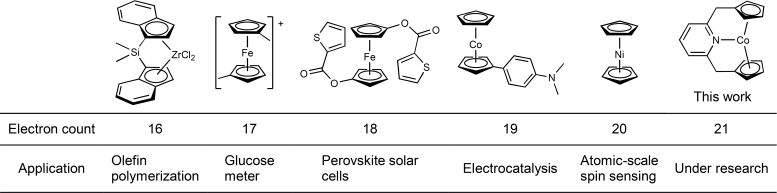
Examples of metallocene chemical compounds, their electron counts, and applications. Credit: Takebayashi et al., 2023
Recent Development at OIST
Dr. Satoshi Takebayashi, a researcher at the Science and Technology Group at the Okinawa Institute of Science and Technology (OIST), together with Dr. Hyung-Been Kang, a scientist at OIST’s Engineering Section, and scientists from Germany, Russia, and Japan, has successfully developed a new metallocene compound at OIST.
The chemical structure of metallocenes can accommodate a variety of electron counts, allowing for the formation of complexes with up to 20 electrons. However, the 18-electron structure is most favored as it is the most stable version.
“Having more than 18 electrons is known to be rare because if you deviate from 18, the chemical bonds of the metallocenes start to elongate, break, and change structure. However, we added two more electrons to a 19-electron metallocene and created a 21-electron metallocene. I think most people didn’t think this was possible, but our 21-electron metallocene is stable in solution and solid states and can be stored for a long time,” Dr. Takebayashi explained.
With this new metallocene, we can potentially create novel materials that can be used for applications in medicine, catalysis, and the energy sector, helping to solve important global problems and improving our quality of life.
Challenges and Collaboration in Research
Because the sandwich structure of metallocenes can easily be altered, the most challenging part of the research was for the scientists to show that the nitrogen had successfully bonded to the cobalt without altering the sandwich structure. They had to rigorously prove that the metallocene was properly bonded to all neighboring carbon atoms and that the nitrogen atom was attached to the cobalt atom. To do this, Dr. Takebayashi organized a strong team of researchers with different specialties and unambiguously showed that all the elements had bonded well.
“This breakthrough would not have been possible without the participation of my collaborators who did substantial work,” Dr. Takebayashi added. Dr. Satoshi Takebayashi, Jama Ariai, Dr. Urs Gellrich, Sergey Kartashov, Dr. Robert Fayzullin, Dr. Hyung-Been Kang, Dr. Takeshi Yamane, Dr. Kenji Sugisaki, and Prof. Kazunobu Sato have coauthored an article published in the journal Nature Communications detailing their discovery.
Looking Ahead
Dr. Takebayashi’s future research will focus on the utilization of the 21-electron metallocene for more applicable science such as catalysis and material science, as well as the discovery of unprecedented organometallic chemistry based on this finding.
Reference: “Synthesis and characterization of a formal 21-electron cobaltocene derivative” by Satoshi Takebayashi, Jama Ariai, Urs Gellrich, Sergey V. Kartashov, Robert R. Fayzullin, Hyung-Been Kang, Takeshi Yamane, Kenji Sugisaki and Kazunobu Sato, 5 September 2023, Nature Communications.
DOI: 10.1038/s41467-023-40557-7






No idea why every atom except Hydrogen has a geometry that looks like it could be 1/8th retro-reflective.
If it was up to me, every Hydrogen nucleus shown in the top figure would be drawn as a full retro-reflector (omni-retroreflector) with eight cube-corner divots instead of just one as is shown for some of the atoms, where Nitrogen and Cobalt show cross-hatching on the planar surfaces resulting from removing a cube-corner, while Carbon does not, but I’m thinking in terms of gravity coupling dependency on thermal considerations, not charge-coupling considerations.