
Photons are elementary particles that serve as the quantum of light and all other forms of electromagnetic radiation. They are unique in that they have zero mass, do not carry electric charge, and always move at the speed of light within a vacuum. Photons exhibit both wave-like and particle-like properties, a dual nature that allows them to be described both as waves of electromagnetic energy and as discrete particles. Credit: SciTechDaily.com
What Are Photons?
Photons are the smallest possible particles of electromagnetic energy and therefore also the smallest possible particles of light. Photons can travel at the speed of light because they have no mass (thanks to relativity). Photons also have no charge.
Photons represent the entire spectrum of electromagnetic radiation. This includes radio waves, gamma-rays, and visible light.
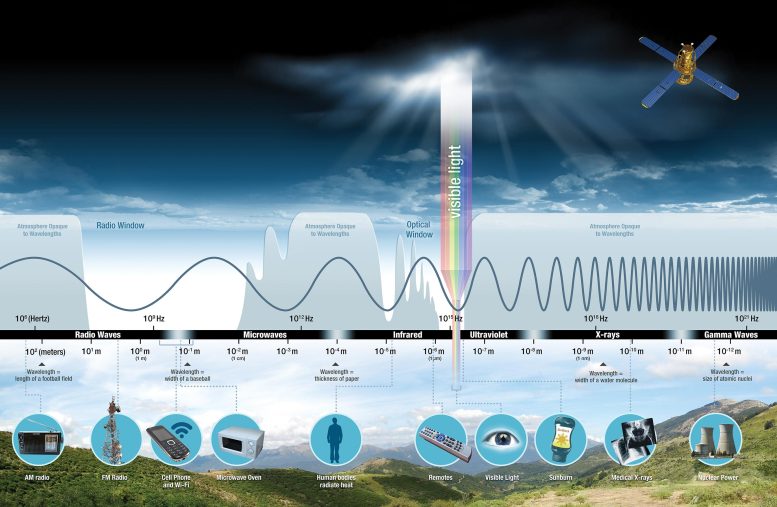
Photons carry electromagnetic energy. This energy includes the visible light we can see and many other types of lower- and higher-energy forms of energy. Credit: NASA
Photons: Wave-Particle Duality
Like many other particles governed by quantum mechanics, photons have the characteristics of both waves and particles. Photons travel in a wave-like fashion, in which the local electric and magnetic field oscillates in intensity, regularly swinging back and forth between high and low energy levels.
The energy in the photons determines the type of electromagnetic radiation the photons transmit. This means low-energy photons carry radio waves. Radio waves are called long-wavelength because the bottoms and crests of the waves are relatively far apart. High-energy photons carry gamma waves. Gamma waves are called short-wavelength because the bottoms and crests of their waves are very close together.
Historical Perspectives on Light
Scientists discovering that photons are both waves and particles was a key part of the development of quantum mechanics. Isaac Newton thought light traveled in particles in part because of how light bounces off surfaces. But Christian Huygens pointed out that light shined through a small hole spread out instead of remaining a spot. This meant that light must be a wave, with light spreading out in a way similar to how water ripples. Huygens’ wave model gained additional support from experiments in the 1800s.
Quantum Mechanics and Photons
In 1900, Max Planck suggested instead that photons must be particles because of the way radiation behaves. This opened the door for Einstein to suggest in 1905 that light is both particles and waves. His explanation is based on the photoelectric effect. This is the way a sheet of metal emits electrons when hit with light. Einstein demonstrated that the intensity of the light shined on the metal is tied to the number of photons in the light. The frequency (or color) of the light is tied to the amount of energy each photon carries.
Fast Facts
- Photons can be absorbed by matter. When this happens, their light is transformed into heat, warming the matter. That’s how sunlight warms up things on Earth.
- Albert Einstein won the Nobel Prize in Physics in 1922 for his work on photons and electromagnetic radiation.
DOE Office of Science: Contributions to Subatomic Particle Research
The DOE Office of Nuclear Physics in the Office of Science supports research to understand all forms of nuclear matter and the subatomic particles that make up atomic nuclei. This research includes unraveling previously unknown properties of atoms and the subatomic particles they are composed of in their natural state. This work has important applications in medicine, commerce, and national defense.
Another area of study is understanding precisely how nuclei are structured depending on the number of protons and neutrons inside them. Still other research focuses on heating nuclei to the temperature of the early universe to understand how they condensed out of the quark-gluon soup that existed at the time.
Separately, the DOE Office of Science operates a series of light sources that use the power of photons for scientific research. These light sources are Office of Science user facilities open to researchers from industry, academia, and the national laboratories.









excellent!
The reason why humans are great is because they are good at thinking.
Please continue to think deeply:
1. What are photons?
2. What is quantum?
3. What is the difference between photons and quantum?
4. What is the spacetime foundation of photons and quantum motion?
5. Is there a correlation between mathematical graphics and physical reality?
6. Why can mathematics become the language of science?
If researchers are really interested in science and physics, you can browse https://zhuanlan.zhihu.com/p/693933588 and https://zhuanlan.zhihu.com/p/595280873.
Mathematics has clearly taught us that topological vortices can create everything in the world via self-organization.
reference
1. https://zhuanlan.zhihu.com/p/684863580.
2. https://zhuanlan.zhihu.com/p/697274113.
3. https://zhuanlan.zhihu.com/p/695926158.
One should read the paper from Willis Lamb (Nobel prize 1955): “anti-photon” showing how bad is the concept of a photon
Splinded