
Quantum mechanics is an essential branch of physics that explains the properties and behaviors of particles at the atomic and subatomic scale. It challenges the principles of classical mechanics with concepts like quantization, where properties like energy exist in discrete units, and wave-particle duality, where particles exhibit both particle and wave characteristics. This theory has resolved many mysteries of the atomic world, leading to significant technological advancements in fields ranging from electronics to medicine.
Quantum mechanics is a key physics theory that explains the unique behavior of atomic and subatomic particles, introducing revolutionary concepts like quantization and wave-particle duality.
Introduction to Quantum Mechanics
Quantum mechanics is the field of physics that explains how extremely small objects simultaneously have the characteristics of both particles (tiny pieces of matter) and waves (a disturbance or variation that transfers energy). Physicists call this the “wave-particle duality.”
Wave-Particle Duality and Quanta
The particle portion of the wave-particle duality involves how objects can be described as “quanta.” A quanta is the smallest discrete unit (such as a particle) of a natural phenomenon in a system where the units are in a bound state. For example, a quanta of electromagnetic radiation, or light, is a photon. A bound state is one where the particles are trapped. One example of a bound state is the electrons, neutrons, and protons that are in an atom.
Quantization in Quantum Mechanics
To be “quantized” means the particles in a bound state can only have discrete values for properties such as energy or momentum. For example, an electron in an atom can only have very specific energy levels. This is different from our world of macroscopic particles, where these properties can be any value in a range. A baseball can have essentially any energy as it is thrown, travels through the air, gradually slows down, and then stops.
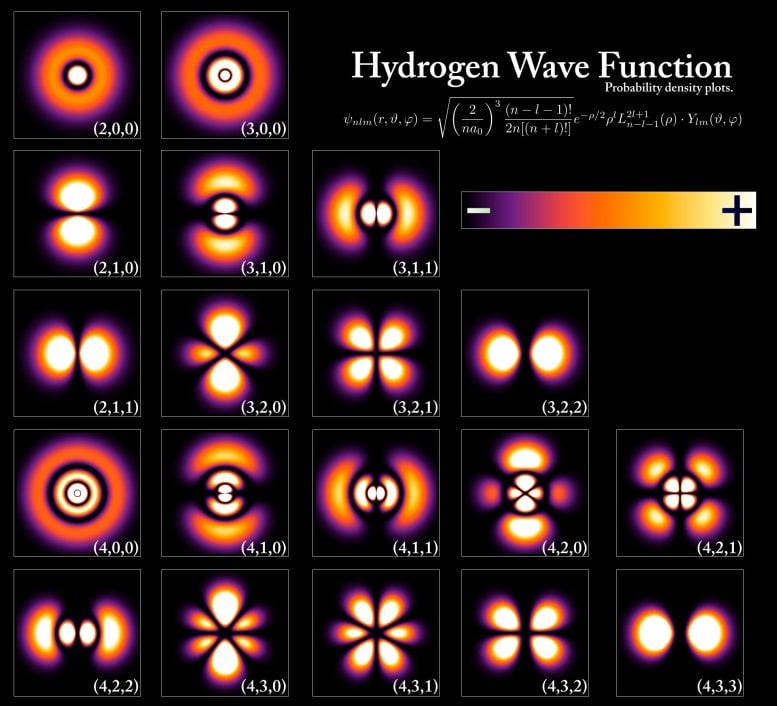
Electrons don’t just travel in circles. Because of quantum mechanics, their positions are described by probabilities that they are in a certain location. These figures describe the probability for electrons in various configurations in a hydrogen atom.
Wave Functions and Quantum World
At the same time, tiny quantized particles such as electrons can also be described as waves. Like a wave in the ocean in our macroscopic world – the world we can see with our eyes – waves in the quantum world are constantly shifting. In quantum mechanics, scientists talk about a particle’s “wave function.” This is a mathematical representation used to describe the probability that a particle exists at a certain location at a certain time with a certain momentum.
Quantum vs. Classical Mechanics
The world of quantum mechanics is very different from how we usually see our macroscopic world, which is controlled by what physicists call classical mechanics. Quantum mechanics grew out of the tremendous progress that physicists made in the early 20th century toward understanding the microscopic world around us and how it differed from the macroscopic world.
Quantum Mechanics and Scientific Progress
As with many things in science, new discoveries prompted new questions. Before this time, scientists thought that light existed as an electromagnetic wave and that electrons existed as discrete, point-like particles. However, this created problems in explaining various phenomena in physics. These include blackbody radiation—the emission of light from objects based on their temperature. Quantum mechanics also helped explain the structure of the atom. It helped make sense of the photoelectric effect, which involves how materials emit electrons when those materials are hit with light of certain wavelengths. By explaining how things can be both particles and waves, quantum mechanics solved these problems.
Impact on Science and Technology
This new knowledge had profound effects in science and technology. Quantum mechanics led to the development of things like lasers, light-emitting diodes, transistors, medical imaging, electron microscopes, and a host of other modern devices. Your cell phone would not exist without the science of quantum mechanics!
Fast Facts
- Many subatomic particles, including the proton, have angular momentum, which is often referred to as “spin.” Medical experts use this property in MRI imaging devices.
- Smartphones contain billions of transistors that work based on the wave nature of electrons, which scientists understand through quantum mechanics.
- Quantum computers and quantum networks are new applications of quantum mechanics that use the quantized nature of particles to store and transfer information.
DOE Office of Science: Contributions to Quantum Mechanics
The Department of Energy Office of Science supports research in technology and science resulting from quantum mechanics. The Office of Science has many programs involved with quantum computing and quantum information science. In addition, modern scientific research supported by the Office of Science takes place within the framework of quantum mechanics.
Accelerator facilities such as the Argonne Tandem Linac Accelerator System (ATLAS) at Argonne National Laboratory, Brookhaven National Laboratory’s Relativistic Heavy Ion Collider (RHIC), and Thomas Jefferson National Accelerator Facility’s Continuous Electron Beam Accelerator Facility all exist to study the properties of the smallest particles of nature. These tiny particles are controlled by the rules of quantum mechanics.
Other facilities that study the quantized nature of particles include Fermi National Accelerator Laboratory, the core U.S. particle physics lab. The Facility for Rare Isotope Beams (FRIB) at Michigan State University exists to study the properties of exotic atomic nuclei.







The wow moment
The yawwhoee moment
Super 😎 🆒️ moment
The littlest makes the ……
Awesome those r just dots….
Right?
Great article of the clearest explanation. Science is life.
I know this site presents quality based articles and additional information, is there any other website which offers such information in quality?|