
Scientists are exploring hydrogen as a clean energy source to combat climate change, focusing on its production through water electrolysis and use in fuel cells for transportation, aligning with the goal of net-zero carbon emissions by 2050.
What Is Hydrogen Energy?
As the effects of climate change take hold, our planet faces record heat waves, unprecedented storms, historic droughts, and wildfires. Scientists have linked these events to greenhouse gases like carbon dioxide in the atmosphere, much of which is produced by human activity.
But what if, instead of releasing harmful greenhouse gases into the environment, our airplanes and cars could run on fuel produced from water, using electricity from the sun or wind? What if this renewable fuel could provide backup power to the electric grid and be purchased from fueling stations across the nation?
In this Science 101 video, scientists Debolina Dasgupta and Nancy Kariuki describe the science, technology, and applications of hydrogen energy. Hydrogen is the simplest chemical element, or type of atom, and an abundance of hydrogen exists within the water on our planet. It is naturally renewed by the water cycle, and when used as fuel, it releases no harmful emissions. For these reasons, hydrogen could play a major role in fostering a cleaner environment and reducing greenhouse gas emissions in sectors ranging from transportation to the grid. Scientists at the U.S. Department of Energy’s Argonne National Laboratory are leveraging world-class facilities and expertise to lower the cost of hydrogen production and develop affordable fuel cells for hydrogen-powered vehicles. They’re also assessing methods of hydrogen production, transport, storage and use to minimize greenhouse gas emissions.
Scientists are working to make this vision a reality using the energy within hydrogen, which promises to play a major role in fostering a cleaner environment and achieving the U.S. goal to attain net-zero carbon emissions by 2050 — in other words, removing carbon from the atmosphere at the same rate it is emitted.
Hydrogen is the simplest chemical element, or type of atom. It consists of just one proton and one electron. It is also the most abundant element, making up around 75% of the known matter in the universe. Vast amounts of hydrogen exist in water and living things.
An abundance of hydrogen exists within the water on our planet, and it is naturally renewed by the water cycle. When used as fuel, it releases no carbon emissions, making it a promising clean energy source. Credit: Argonne National Laboratory
The hydrogen molecule, consisting of two hydrogen atoms, can be used to produce carbon-free energy. Hydrogen molecules carry a lot of energy; a pound of hydrogen contains almost three times the energy of a pound of gasoline or diesel.
However, hydrogen molecules are not abundant on Earth, making up less than 0.0001% of our atmosphere. Because of this, hydrogen must be produced from other substances that contain it. The most common way to produce hydrogen that doesn’t use fossil fuels is to split water (H2O) into hydrogen (H2) and oxygen (O2) using electricity. This process, called water electrolysis, is a promising option for carbon-free hydrogen production since the electricity can be sourced from nuclear or renewable energy, such as wind and solar. Scientists and engineers are working to improve and lower the cost of hydrogen produced by water electrolysis.
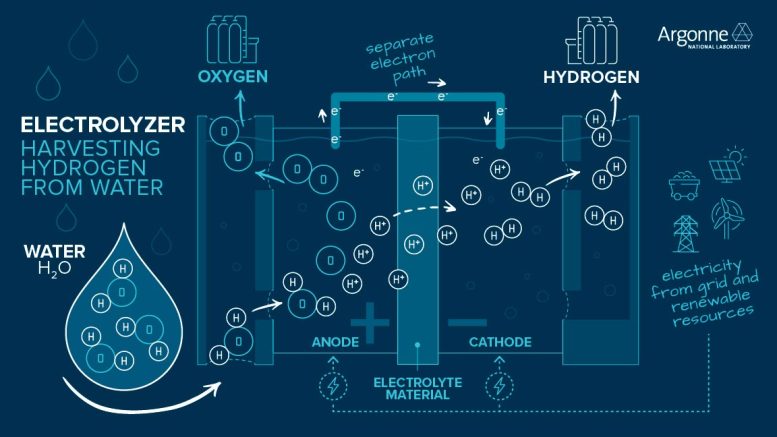
In electrolysis, water splits at the anode to form oxygen, hydrogen ions, and electrons. An electrolyte material allows hydrogen ions through, but forces electrons to flow separately to the cathode, where the two recombine to form hydrogen gas for use as fuel. Credit: Argonne National Laboratory
They are also developing methods that convert solar energy and water directly to hydrogen by harnessing and mimicking biological processes like photosynthesis.
There are several ways to use hydrogen for energy once it is produced. The most prominent is in fuel cells, which convert the chemical energy stored in hydrogen and oxygen into electricity. Unlike with gasoline-fueled engines, there are no harmful emissions like carbon dioxide. And unlike with batteries, fuel cell systems don’t require lengthy downtimes for recharging. They are refueled like gasoline-fueled engines, but with hydrogen.
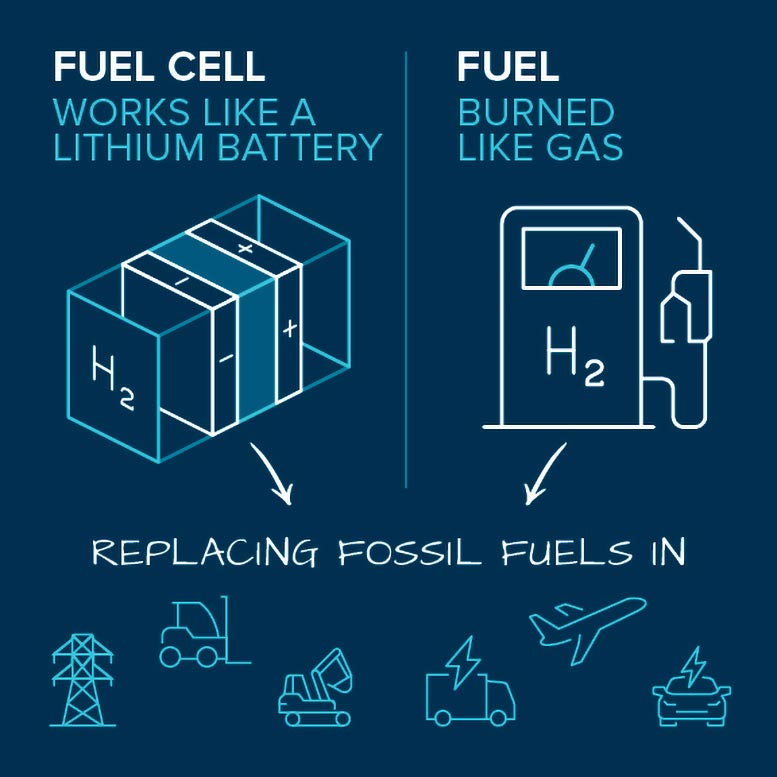
Hydrogen can be used in fuel cells or burned as fuel in engines. Scientists and engineers are working to improve these technologies, which could replace the use of fossil fuels in transportation and the grid. Credit: Argonne National Laboratory
A type of hydrogen fuel cell being developed for cars, trucks, forklifts, buses, ships, and trains splits hydrogen molecules into electrons and protons. The electrons are forced to flow through an electric circuit, creating a supply of usable electricity. Meanwhile, the protons are able to pass through a membrane, ultimately recombining with the electrons and reacting with oxygen molecules from the air to produce water, the only emission.
Scientists at the U.S. Department of Energy’s Argonne National Laboratory are leveraging world-class facilities and expertise to advance hydrogen science and technology. Our researchers are lowering the cost of hydrogen production, developing affordable fuel cells for hydrogen-powered vehicles. They’re also assessing methods of hydrogen production, transport, use, and storage to minimize greenhouse gas emissions.









This article obfuscates like an advertisement; electrolysis takes energy, probably from burning coal or natural gas. Let me simplify it more.
Using electricity, you can split H2O water into 2 hydrogen H and an oxygen O. If you add the oxygen back to make H2O, you get most of that energy back and released (think Hinderburg blimp exploding), and can move a car with it, and only water comes out as exhaust. There’s other ways of doing it, like a fuel cell getting electricity back out, but same basic idea. It’s like a rechargeable battery, using electricity to turn a chemical into another chemical, and then the reaction of turning it back releases most of the energy. You still lose energy at every step, but the advantage of hydrogen is you get way more energy from a jug of hydrogen than a jug of rechargable batteries. The main challenge, apart from getting the electricity to start with, is it needs a very special jug, or again, Hindenburg explosion.
It makes more sense if you think of hydrogen as energy storage instead of a fuel or “clean energy source”, as making it takes more energy than you get out of it. Even our best batteries have terrible energy density, so hydrogen is a better obvious clean simple answer. There are new ways of making hydrogen coming, but of course, if you’re burning fossil fuels to make the hydrogen, it’s way more efficient to skip all the hydrogen steps and just burn the fossil fuel. I like hydrogen energy, but we have to be realistic.
” …, and when used as fuel, it releases no harmful emissions.”
Not so! Water vapor is a more powerful greenhouse gas than carbon dioxide. However, water vapor is usually ignored because it condenses out quickly (rain/snow) and is therefore,relatively constant in concentration. However, it is also a byproduct of the use of fossil fuels. With a ‘hydrogen economy,’ that is ALL that is produced, replacing the CO2 from fossil fuel combustion. The article doesn’t address how the waste water will be handled. If, as is the case with exhaust from internal combustion engines (ICE), it is released to the air, then the relative humidity will be increased, at least in cities, leading to local and regional warming. It will also result in increasing the heat index, increasing the demand for air conditioning and electricity. It will probably result in increasing the growth of mold on buildings and other structures, and increase the rate of rusting of exposed steel. If, in the Winter, it condenses onto the roads and freezes, it will make them slippery and increase the number and severity of accidents. If the water vapor from fuel cells or ICE vehicles is first condensed onboard before being released to drip on the roads, it will be worse than exhausting the vapor. Even wet roads in the Summer will increase braking distance, leading to more accidents. Low-level fog may become more common, impeding visibility, again resulting in increased accidents.
One solution to the problems outlined above is to retain the water onboard, and periodically drain it. However, that means the weight of the vehicle will continually increase between drainings, reducing the fuel economy. Increasing the weight will increase the braking distance and increase brake-lining wear, tire wear, and pavement damage. I don’t imaging that drivers will be thrilled with the requirement to dump their water load every time they fill up with hydrogen.
Once again, the technologists focus on the technical problems without looking at the Big Picture. They also make unexamined assumptions such as thinking that something that isn’t a problem today won’t become a problem in the future.
Incidentally, if the auto’ manufacturers would be willing to add to the complexity and cost of cars to handle the water vapor problem with a ‘hydrogen economy,’ they could similarly add CO2 scrubbers to cars running on gasoline. The technology exists. However, that would lead to increased maintenance and costs that the consumers probably would not be happy with. The whole problem is really more of an economics problem than one of needing a breakthrough in technology.
That reads a little like my style of comments, where I’d satirically panic about water exhaust. It’s all true. Hilarious, until I remember we’re supposed to panic to eliminate CO2. We may as well panic about water also. Not to mention, it’ll be pure water like distilled, acidic pH 5.6 to 7 as it absorbs atmospheric gasses, forming carbonic acid with no dissolved solids, and hot, much more aggressive than rain at dissolving roads and potentially more corrosive.
I like the idea of cars having a pure water tap inside, but it’ll mean more bathroom stops.
With hydrogen things could go boom a lot. It’s difficult to store and keep. Producing it is energetically inefficient. Water vapour that is emitted during combustion is more potent than CO2 in greenhouse terms.
It’s not gonna be the magic wand to solve the supposed climatological problems with.