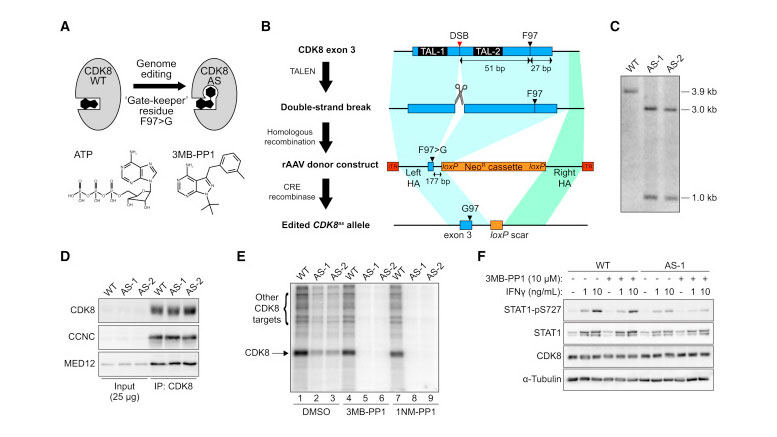
Engineering and Validation of CDK8as/as HCT116 Cells (A) Cartoon depicting the creation of analog-sensitive CDK8-AS by altering the gatekeeper residue in the kinase active site. Structures of ATP and the analog 3MB-PP1 are shown for reference. (B) Outline of genome-editing strategy to generate CDK8as/as HCT116 cells. Each round entailed generation of a DNA double-strand break (DSB) in exon 3 of CDK8, using a transcription activator-like effector nuclease (TALEN) pair, followed by homologous recombination with a recombinant adeno-associated virus (rAAV)-based repair donor construct containing the F97G mutation and a loxP-flanked neomycin resistance (NeoR) cassette, selection for resistance, and finally removal of the NeoR cassette using transient expression of CRE recombinase. TAL-1 and TAL-2, TALEN-binding sites; HA, homology arm; ITR, inverted terminal repeat. (C) Southern blot hybridization analysis of AvrII-digested genomic DNA from WT and two independent homozygous CDK8as/as clones (AS-1 and AS-2), using a probe spanning the novel AvrII restriction site introduced along with the F97G mutation in CDK8 exon 3. Fragment sizes in kilobases are indicated at right. (D) Western blot analysis of CDK8, Cyclin C (CCNC), and MED12 levels for inputs (2.5%) and CDK8 immunoprecipitations from WT and AS lysates. (E) In vitro kinase assay with CDK8 immunoprecipitated material, as in (D), showing labeling of proteins with 32P-ATP in the presence of vehicle (DMSO) or the ATP analogs 3MB-PP1 (10 μM) and 1NM-PP1 (10 μM). Arrows indicate bands representing phosphorylation of CDK8 itself, or additional proteins present in the immunoprecipitation. (F) Western blot showing levels of S727-phosphorylated STAT1 (STAT1-pS727), total STAT1, and CDK8 in HCT116 WT or CDK8 AS-1 cell lysates following treatment with interferon gamma (IFNγ) and/or 10 μM 3MB-PP1.
In a newly published study, researchers pinpoint a way to restrict the ability of cancer to use glucose for energy.
Cancer cells consume exorbitant amounts of glucose, a key source of energy, and shutting down this glucose consumption has long been considered a logical therapeutic strategy. However, good pharmacological targets to stop cancers’ ability to uptake and metabolize glucose are missing. In a new study published in Cell Reports, a team of University of Colorado Cancer Center researchers, led by Matthew Galbraith, Ph.D., and Joaquin Espinosa, Ph.D., finally identifies a way to restrict the ability of cancer to use glucose for energy.
Over-expression of the gene CDK8 is linked to the development of many cancers including colorectal cancer, melanoma, and breast cancer, where it regulates pathways that drive the growth and survival of cancer cells. Although a number of drugs aimed at blocking CDK8 activity are currently being developed, it is not yet clear how effective they are at treating various cancers. Galbraith and Espinosa have been working to better understand the role of CDK8 in cancer biology in the hopes of aiding the introduction of CDK8-based therapies as cancer treatments.
Their most recent study, which was funded in part by the Cancer League of Colorado and the Mary Miller and Charlie Fonfara-Larose Leukemia in Down Syndrome Fund, demonstrates that CDK8 plays a critical role in allowing cancer cells to use glucose as an energy source.
The finding takes place against the backdrop of the tissue conditions in which tumors grow – as cancer cells rapidly multiply, their growth often outstrips their blood supply, leading to depletion of oxygen (i.e. hypoxia) and other nutrients such as glucose. In 2013, the group published a paper showing that CDK8 is important for activation of many genes switched on in hypoxic conditions. During adaptation to these conditions, cancer cells must alter their metabolism to consume more glucose through a process called glycolysis. In fact, many cancer cells have permanent increases in glycolysis, maintained even in conditions of plentiful oxygen, a phenomenon known as the Warburg effect, which was described as far back as 1924. Consequently, many cancers are heavily dependent on glucose metabolism for their growth and survival. This is true to the point that doctors use glucose isotopes and PET scans to pinpoint the exact location of a tumor and its metastases within the human body – where there are abnormally high levels of glucose being used, chances are there is a cancerous growth.
When Galbraith used a sophisticated chemical genetics approach to specifically switch off CDK8 activity in colorectal cancer cells, he saw that the cells failed to activate glycolysis genes and took up much less glucose. He confirmed this in experiments showing that blocking CDK8 activity leads to a lower rate of glucose use.
“Because of this role of CDK8 in glycolysis, I reasoned that the cells with impaired CDK8 activity should be more susceptible to drugs that block glycolysis,” Galbraith says. Sure enough, treating cancer cells with drugs that block both CDK8 and glycolysis slowed their growth more effectively than either approach alone.
“These are very exciting discoveries. The Warburg effect and consequent addiction to glucose is a hallmark of cancerous tissues, something that distinguishes cancer cells from most normal tissues. Therefore, combining drugs that block CDK8 activity with those that block glycolysis may enable specific targeting of cancer cells without harmful effects on normal cells,” says Espinosa, the paper’s senior author.
The team was recently awarded a grant from the Denver chapter of Golfers Against Cancer to advance their findings through pre-clinical research in mouse models, a necessary step to test the clinical value of this new strategy targeting CDK8 and glucose metabolism.
Reference: “CDK8 Kinase Activity Promotes Glycolysis” by Matthew D. Galbraith, Zdenek Andrysik, Ahwan Pandey, Maria Hoh, Elizabeth A. Bonner, Amanda A. Hill, Kelly D. Sullivan and Joaquín M. Espinosa, 7 November 2017, Cell Reports.
DOI: 10.1016/j.celrep.2017.10.058




Be the first to comment on "Scientists Reveal a New Way to Shut Down Cancer Cells’ Ability to Consume Glucose"