Barth syndrome is a rare genetic disorder that primarily affects males, characterized by abnormalities in mitochondrial function. It leads to muscle weakness, particularly in the heart, as well as growth delay, and an increased risk of infection. The syndrome is caused by a mutation in the tafazzin gene, which disrupts the normal production of cardiolipins, essential lipids in the mitochondrial membrane.
Patrick van der Wel, an associate professor at the Zernike Institute for Advanced Materials at the University of Groningen in the Netherlands, specializes in Solid State NMR. His expertise enables him to analyze the connections between atoms in various materials, offering insights into their structural composition.
This technique is used to study new materials for solar panels, but also biomaterials. In a study recently published in Nature Metabolism, Van der Wel contributed to the measurements that helped discover the root cause of a deadly metabolic illness called Barth syndrome, which could be a step toward a cure.
In Barth syndrome, something is wrong with the energy factories of the cells, the mitochondria. This leads to the weakening of muscles, including the heart. As there is no known cure or treatment, patients — there are just a few hundred families worldwide in which this Barth syndrome is present — often die prematurely.
The syndrome is caused by a mutation in a gene called tafazzin, which produces an enzyme that plays a role in the shaping of so-called cardiolipins, molecules that are unique to mitochondria. But so far, it hasn’t been clear exactly what is going wrong.

This is prof. dr. Patrick van der Wel, who used solid state NMR to confirm the root cause of Barth syndrome. Credit: University of Groningen
Energy factories
Van der Wel has a long-standing collaboration in studying mitochondrial lipids together with scientists at the University of Pittsburgh—led by professor Valerian Kagan— a place where Van der Wel once worked in the past. His co-workers came up with a hypothesis for the root cause of Barth syndrome: faulty cardiolipin molecules would bind to a protein called cytochrome c, and form a complex that oxidizes lipids in the mitochondria.
This would then damage these energy factories, causing them to malfunction. Through genetics, computer simulations, biochemical experiments, and Van der Wel’s solid-state NMR measurements, the international team set out to prove this scenario.
Fruit flies
‘We used a number of different techniques to investigate this complex, one of which was solid-state NMR’, says Van der Wel. This NMR technology is related to the more familiar MRI scans that are made in hospitals. In brief, this technology makes use of radio frequency signals that make atoms vibrate – a bit like striking a tuning fork. Every atom produces a unique ‘sound’, but this is modified when they connect to other atoms. Consequently, this technique shows Van der Wel which atoms in a material are connected, and also what the structure of a molecule is.
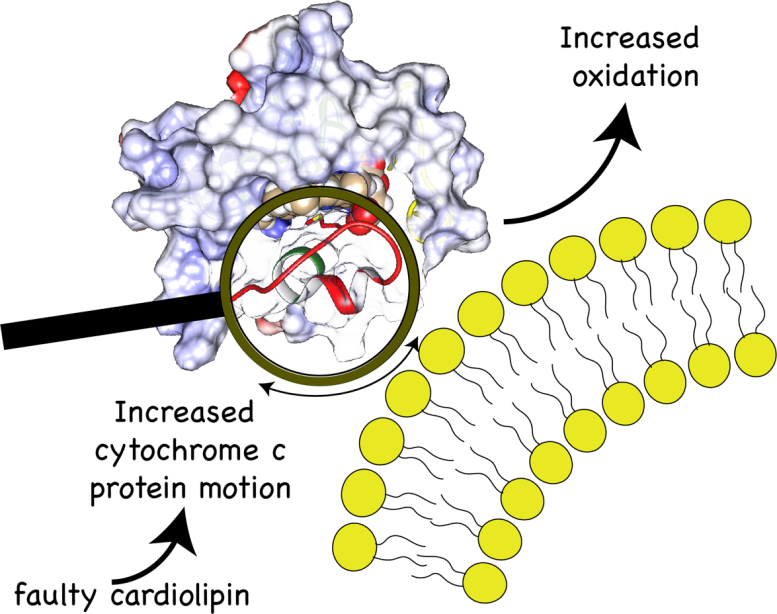
Cardiolipin with a faulty shape makes the cytochrome c protein less rigid. This results in the formation of an oxidizing complex. Credit: Patrick van de Wel, University of Groningen
In this way, he was able to demonstrate how the faulty shape of the cardiolipin could facilitate the formation of an oxidizing complex. ‘A great thing about solid state NMR is that it also allows you to see the dynamics of molecules’, says Van der Wel. ‘Normally, cytochrome c is a rigid molecule, but in this complex, it becomes floppy. This structural change causes the toxic oxidation of lipids in the mitochondrial membrane.’ Moreover, Van der Wel was able to show how and where a molecule that improves the condition of fruit flies with Barth syndrome interacts with the cytochrome c protein, a result that was also supported by the other techniques used in the study.
Joint effort
As a result, the scientists have demonstrated both the probable root cause of Barth syndrome, and a potential way to treat the disease in an animal model. The road to a treatment for patients is still very long, but Van der Wel and his colleagues have made great progress. The next step is to analyze the faulty complex in more detail. This would help them to, for example, design a drug that could be used in humans.
Van der Wel points out that this study was a real joint effort. ‘Our paper is the result of the combined expertise of scientists from all over the world.’ Furthermore, the project shows how fundamental science can help shed light on a very practical problem, such as the mechanism for a disease. ‘Solid state NMR is one of the few methods available to see what the complex of a cardiolipin with cytochrome c looks like. This means that our materials science lab can provide valuable insights into biomedical problems.’
Reference: “Anomalous peroxidase activity of cytochrome c is the primary pathogenic target in Barth syndrome” by Valerian E. Kagan, Yulia Y. Tyurina, Karolina Mikulska-Ruminska, Deena Damschroder, Eduardo Vieira Neto, Alessia Lasorsa, Alexander A. Kapralov, Vladimir A. Tyurin, Andrew A. Amoscato, Svetlana N. Samovich, Austin B. Souryavong, Haider H. Dar, Abu Ramim, Zhuqing Liang, Pablo Lazcano, Jiajia Ji, Michael W. Schmidtke, Kirill Kiselyov, Aybike Korkmaz, Georgy K. Vladimirov, Margarita A. Artyukhova, Pushpa Rampratap, Laura K. Cole, Ammanamanchi Niyatie, Emma-Kate Baker, Jim Peterson, Grant M. Hatch, Jeffrey Atkinson, Jerry Vockley, Bernhard Kühn, Robert Wessells, Patrick C. A. van der Wel, Ivet Bahar, Hülya Bayir and Miriam L. Greenberg, 23 November 2023, Nature Metabolism.
DOI: 10.1038/s42255-023-00926-4







Be the first to comment on "Scientists Uncover Clue To Treat Deadly Hereditary Illness"