Nuclear physicists at RIKEN have successfully created an extremely neutron-rich isotope of sodium, 39Na, previously predicted by many atomic nuclei models to be non-existent. This discovery has significant implications for our understanding of atomic nuclei structure and the astrophysical processes that form heavier elements on Earth.
Nuclear physicists have made the most neutron-rich form of sodium yet, which will help reveal more about the complex world of nuclei.
Physicists at RIKEN have created an exceptionally neutron-rich sodium isotope, 39Na, which was previously believed to be impossible. This breakthrough has major implications for understanding atomic nuclei structure and the creation of Earth’s heavier elements.
In extremely neutron-rich form of the element sodium—which many models of atomic nuclei predict shouldn’t exist—has been created by nuclear physicists at RIKEN for the first time[1].
If you made table salt from this super-heavy version of sodium—and the most neutron-rich isotope of chlorine, salt’s other constituent—it would taste and behave like normal salt, except it would be roughly 1.6 times heavier, says nuclear physicist Toshiyuki Kubo.
But far more than being a scientific curiosity, this finding has important implications for theories on the structure of atomic nuclei. This knowledge in turn informs our understanding of the astrophysical processes that form Earth’s heavier elements.
In terms of nuclear theory, the finding provides a vital reference point for tweaking models of neutron-rich nuclei and for assessing their accuracy, explains Kubo. Theoretical studies of neutron-rich nuclei involve extremely complicated calculations, and theoretical physicists have so far only been able to precisely model more stable nuclei with few neutrons. This finding could help refine calculations for nuclei with more neutrons.
This in turn has implications for our understanding about the origins of heavier elements. For example, the nuclear astrophysical processes that create Earth’s heavy metals are thought to be the result of the huge amounts of energy produced by the merger of two neutron stars or collisions of neutron stars and black holes. The gas and dust released eventually contribute to the rare materials of planets, such as Earth. However, the exact processes that produce heavy metals have long been debated.
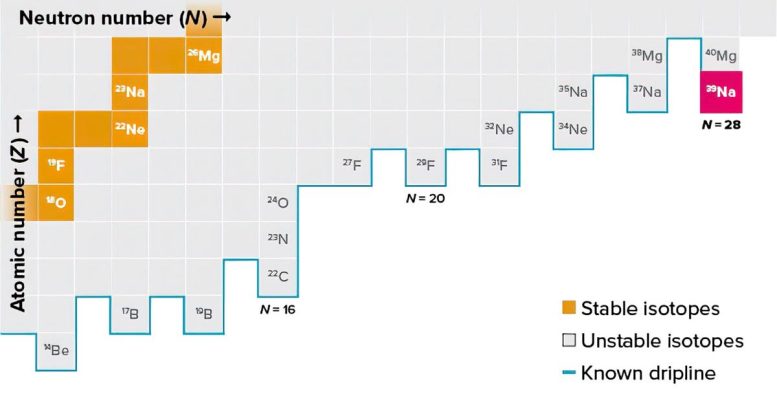
A new square on the drip line: Each square indicates an isotope, with the number of protons increasing as the squares move vertically upward and the number of neutrons increases horizontally to the right. The known existence limit, the neutron ‘drip line’, is indicated by a thick blue line. Sodium-39 (39Na) in red has 11 protons and 28 neutrons, giving it a mass number of 39. Its recent discovery by RIKEN researchers has seen it added to the drip line. Credit: © 2023 RIKEN
Packing neutrons into sodium
Each of the 118 known elements has a fixed number of protons (11 in the case of sodium), but the number of neutrons in its nuclei has can vary, notes Kubo. The only stable form of sodium contains 12 neutrons, whereas the newly discovered one has more than double at 28, which is two more neutrons than the previous record holder for the most-neutron-rich isotope of sodium, 37Na, which was discovered more than 20 years ago.
Since neutrons are electrically neutral, they don’t influence an atom’s electrons and hence have no effect on the element’s chemistry. Thus, atoms of the same element that contain different numbers of neutrons—known as isotopes—are chemically indistinguishable.
The impetus to search for the new form of sodium (called 39Na because its nucleus contains 39 neutrons and protons) came from a previous experiment, when a team led by Kubo at the RIKEN Nishina Center for Accelerator-Based Science stumbled upon what appeared to be one nucleus of 39Na. “We were very surprised at this one event,” recalls Kubo. “And so, we decided to revisit the search for 39Na in our present experiment.”
In the latest experiment, they put the existence of 39Na beyond all doubt by creating nine nuclei of the isotope in a two-day run at RIKEN’s Radioactive Isotope Beam Factory—one of only about three nuclear facilities in the world currently capable of producing such nuclei.
Isotope hunter
It’s far from the first time that Kubo has helped to create a new isotope during his four-decade-long career. “Actually, I’ve been involved in discoveries of about 200 new isotopes or so,” he says. “I really enjoy creating and observing what nobody has ever seen before.”
But the discovery of 39Na, has special significance for him, not least because many nuclear models predict that it shouldn’t exist. “The discovery makes a significant impact on nuclear mass models and nuclear theories that address the edge of the nuclear stability, because it provides a key benchmark for their validation,” explains Kubo. For example, Kubo notes that a model developed by a Japanese team in 2020 correctly predicted the existence of 39Na and its predictions for other isotopes have been on target[2], boosting its credibility.
Tracking the drip line
One reason the discovery is important is because 39Na could well be the most neutron-rich version of sodium that it is possible to produce. Nuclear physicists are particularly interested in determining the maximum number of neutrons an element can have before it starts leaking neutrons—a quantity known as the neutron drip line when plotted on a table of nuclei. The location of this limit provides a key benchmark to not only nuclear theories, but also nuclear mass models that play a key role in theories of nucleosynthesis.
But it is extremely difficult to ascertain the drip line for an element—nuclear physicists have so far only succeeded in determining it up to the tenth element in the periodic table, neon, which means they still have 108 more elements to go.
One reason why it is hard to measure the dripline is because of the tiny possibilities involved in creating nuclei that lie close to limits of stability. Another difficulty is that it is extremely challenging to rule out the existence of other nuclei that have even more neutrons. Kubo says that it may be possible to make 41Na, in which case it would become the dripline for sodium, although he notes that the 2020 Japanese model predicts that 39Na is the drip line.
Next Kubo and his team intend to attempt to experimentally determine the dripline for magnesium—one element up from sodium. They also want to probe the structure of 39Na. “We would like to directly study the nuclear structure that allows 39Na to exist,” Kubo explains.
References:
- “Discovery of 39Na” by D. S. Ahn et al., 14 November 2022, Physical Review Letters.
DOI: 10.1103/PhysRevLett.129.212502 - “The impact of nuclear shape on the emergence of the neutron dripline” by Naofumi Tsunoda, Takaharu Otsuka, Kazuo Takayanagi, Noritaka Shimizu, Toshio Suzuki, Yutaka Utsuno, Sota Yoshida and Hideki Ueno, 4 November 2020, Nature.
DOI: 10.1038/s41586-020-2848-x









I am wondering how the new sodium isotope will affect medical solutions like Na Cl(fluid and electrolytes)and albumin and hyponatremia?
Julie M. Dochtermann MSN
Most likely not at all.
Sich extreme isotopes usually have extremely short half life times, and you can’t really deliver medicine in microseconds (the taste comment in the article was misleading in that it considered a counterfactual).
Na=Gd and we are all eyes of the beholder GOD ALMIGHTY
Interesting isotope crystal with thought on energy storage or informational storage what if the isotope were in abundance in usable structures.
The first 4 paragraphs basically say the same thing over and over. Fire your editor.
The half life of the new sodium isotope is not mentioned.