
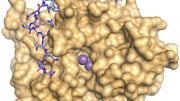
Researchers have developed a sunlight-powered process using copper and nanocrystalline carbon nitride to efficiently convert CO2 into methanol, marking a significant step towards sustainable fuel production and CO2 reduction. The picture above depicts the reactor where the catalyst is tested for turning CO2 to methanol. Credit: University of Nottingham
Researchers have successfully transformed CO2 into methanol by shining sunlight on single atoms of copper deposited on a light-activated material, a discovery that paves the way for creating new green fuels.
An international team of researchers from the University of Nottingham’s School of Chemistry, University of Birmingham, University of Queensland, and University of Ulm have designed a material, made up of copper anchored on nanocrystalline carbon nitride. The copper atoms are nested within the nanocrystalline structure, which allows electrons to move from carbon nitride to CO2, an essential step in the production of methanol from CO2 under the influence of solar irradiation. The research has been published in the Sustainable Energy & Fuels journal of the Royal Society of Chemistry.
The Challenge of Efficiency and Selectivity
In photocatalysis, light is shone on a semiconductor material that excites electrons, enabling them to travel through the material to react with CO2 and water, leading to a variety of useful products, including methanol, which is a green fuel. Despite recent progress, this process suffers from a lack of efficiency and selectivity.
Carbon dioxide is the greatest contributor to global warming. Although, it is possible to convert CO2 to useful products, traditional thermal methods rely on hydrogen sourced from fossil fuels. It is important to develop alternative methods based on photo- and electrocatalysis, taking advantage of the sustainable solar energy and abundance of omnipresent water.
Nanoscale Control for Improved Catalysis
Dr Madasamy Thangamuthu, a research fellow in the School of Chemistry, University of Nottingham, who co-led the research team, said: “There is a large variety of different materials used in photocatalysis. It is important that the photocatalyst absorbs light and separates charge carriers with high efficiency. In our approach, we control the material at the nanoscale. We developed a new form of carbon nitride with crystalline nanoscale domains that allow efficient interaction with light as well as sufficient charge separation.”
The process of CO2 conversion to methanol (fuel) by light. Credit: University of Nottingham
The researchers devised a process of heating carbon nitride to the required degree of crystallinity, maximizing the functional properties of this material for photocatalysis. Using magnetron sputtering, they deposited atomic copper in a solventless process, allowing intimate contact between the semiconductor and metal atoms.
Surprising Efficiency Gains
Tara LeMercier, a PhD student who carried out the experimental work at the University of Nottingham, School of Chemistry, said: “We measured the current generated by light and used it as a criterion to judge the quality of the catalyst. Even without copper, the new form of carbon nitride is 44 times more active than traditional carbon nitride. However, to our surprise, the addition of only 1 mg of copper per 1 g of carbon nitride quadrupled this efficiency. Most importantly the selectivity changed from methane, another greenhouse gas, to methanol, a valuable green fuel.”
Professor Andrei Khlobystov, School of Chemistry, University of Nottingham, said: “Carbon dioxide valorization holds the key for achieving the net-zero ambition of the UK. It is vitally important to ensure the sustainability of our catalyst materials for this important reaction. A big advantage of the new catalyst is that it consists of sustainable elements – carbon, nitrogen, and copper – all highly abundant on our planet.”
This invention represents a significant step towards a deep understanding of photocatalytic materials in CO2 conversion. It opens a pathway for creating highly selective and tuneable catalysts where the desired product could be dialed up by controlling the catalyst at the nanoscale.
Reference: “Synergy of nanocrystalline carbon nitride with Cu single atom catalyst leads to selective photocatalytic reduction of CO2 to methanol” by Tara M. LeMercier, Madasamy Thangamuthu, Emerson C. Kohlrausch, Yifan Chen, Craig T. Stoppiello, Michael W. Fay, Graham A. Rance, Gazi N. Aliev, Wolfgang Theis, Johannes Biskupek, Ute Kaiser, Anabel E. Lanterna, Jesum Alves Fernandes and Andrei N. Khlobystov, 6 March 2024, Sustainable Energy & Fuels.
DOI: 10.1039/D4SE00028E
This work is funded by the EPSRC Programme Grant ‘Metal atoms on surfaces and interfaces (MASI) for sustainable future’ www.masi.ac.uk which is set to develop catalyst materials for the conversion of three key molecules – carbon dioxide, hydrogen, and ammonia – crucially important for economy and environment. MASI catalysts are made in an atom-efficient way to ensure sustainable use of chemical elements without depleting supplies of rare elements and making most of the earth’s abundant elements, such as carbon and base metals.






Be the first to comment on "Sunlight to Methanol: Revolutionary CO2 Conversion Achieved With Copper and Carbon Nitride"