
Nuclear clocks, which are even more precise than atomic clocks, could offer scientists new avenues to explore fundamental forces of the universe. An international team, including LMU researchers, has made significant progress toward this, accurately characterizing the excitation energy of thorium-229, the element poised to be the timekeeping component in nuclear clocks. (Artist’s Concept.)
Nuclear clocks could potentially enable scientists to delve into the universe’s fundamental forces in future research endeavors. A critical step forward in this field has been taken by researchers at LMU as part of an international collaboration.
Atomic clocks provide measure time so precisely that they only gain or lose less than one second every 30 billion years. However, with so-called nuclear clocks, it would be possible to measure time even more accurately. Additionally, they could provide scientists with a more profound understanding of fundamental physical phenomena.
“We’re talking about the forces that hold the world together at its core,” says LMU physicist Professor Peter Thirolf, who has been researching nuclear clocks for many years. In contrast to conventional atomic clocks, this type of clock would register forces inside the atomic nucleus.
“This would open up a whole range of research fields that could never be investigated with atomic clocks,” adds Thirolf’s colleague Dr. Sandro Kraemer, who played a major role in driving the project forward while completing his doctorate at KU Leuven in Belgium.
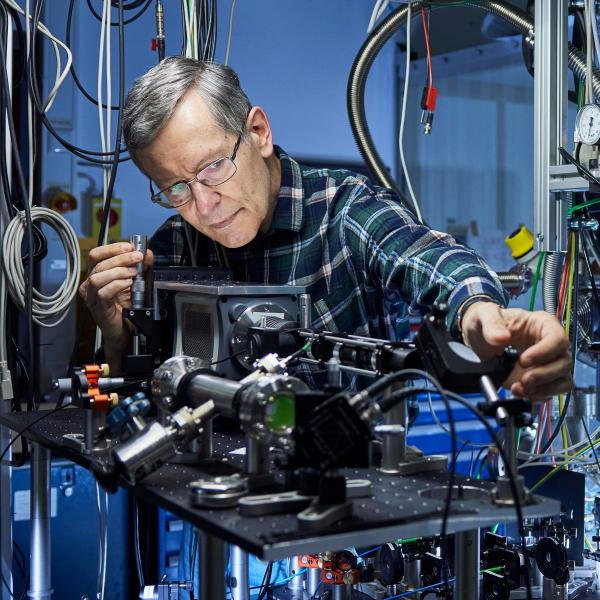
Peter Thirolf has been researching nuclear clocks for many years. Credit: Stephan Höck / LMU
In the race for nuclear time, Thirolf and Kraemer are in the leading pack. Working at the Chair of Experimental Physics in Garching, the two scientists have now made an important advance on the road to the first nuclear clock as part of an international team.
As they report in the journal Nature, they have managed to characterize the excitation energy of thorium-229 with great precision thanks to a new experimental approach. This atomic nucleus is to be used as the timekeeping element of nuclear clocks in the future. Precise knowledge of what frequency it needs for excitation is crucial for the feasibility of the technology.
The innermost clock
For a clock, you need something that periodically oscillates and something that counts the oscillations. A grandfather clock has a mechanical pendulum, the oscillations of which are registered by the clock’s mechanism. In atomic clocks, the atomic shell functions as the timekeeper. Electrons are excited and switch back and forth between high and low energy levels. Then it is a matter of counting the frequency of light particles emitted by the atom when the excited electrons fall back into their ground state.
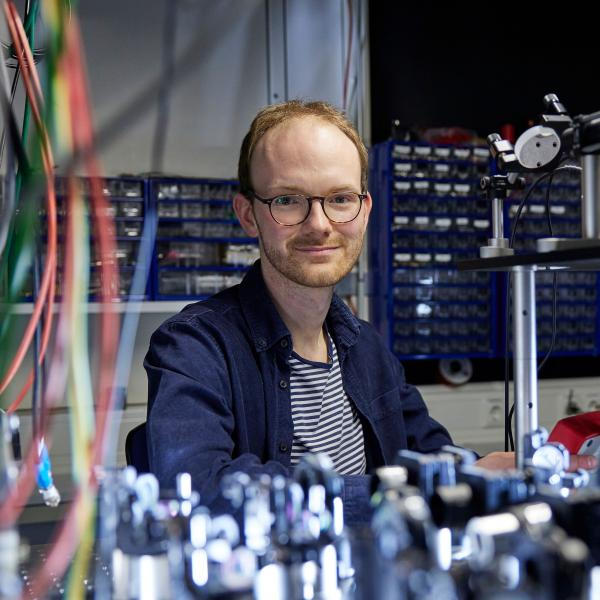
Sandro Kraemer has been researching the nuclear clock as part of his Ph.D. and is now continuing his work at LMU. Credit: Stephan Höck / LMU
In nuclear clocks, the basic principle is very similar. In this case, we penetrate to the nucleus of the atom, where various energy states can also be found. If we managed to excite them precisely with a laser and measure the radiation emitted by the nucleus when falling back into its ground state, then we would have a nuclear clock. The difficulty is that of all atomic nuclei known to science, there is only one that could lend itself to this purpose: thorium-229. And even that was purely theoretical for a long time.
A nucleus like no other
What makes thorium-229 so special is that its nucleus can be put into an excited state using a relatively low light frequency – a frequency just about obtainable with UV lasers. Research stalled for 40 years, because although scientists suspected that an atomic nucleus with the right characteristics exists, they were unable to experimentally confirm this hypothesis.
And then in 2016, Thirolf’s research group at LMU made a breakthrough when they directly confirmed the excited state of the nucleus of thorium-229. This fired the starting gun on the race for the nuclear clock. In the meantime, many groups worldwide have taken up the topic.
To get a clock going, the timekeeping element and the clockwork need to be perfectly attuned to each other. In the case of the nuclear clock, this means that you need to know at what exact frequency the atomic nucleus of thorium-229 oscillates. Only then can you develop lasers that excite exactly this frequency.

Only one known atomic nucleus has the right properties for the development of a nuclear clock: thorium-229. To make the clock tick, one has to find out the exact frequency of light with which it can be excited. Credit: Stephan Höck / LMU
“You can picture it as being like a tuning fork,” explains Kraemer. “As a musical instrument tries to match the frequency of the tuning fork, so the laser tries to hit the frequency of the thorium nucleus.”
If you were to try out all possible frequencies with different lasers, it would take forever. Not to mention that lasers would have to be laboriously developed first in the corresponding UV light spectrum. To narrow down the range in which the oscillation frequency of thorium-229 lies, the researchers, therefore, took a different tack. “Nature is sometimes merciful and offers us various routes,” says Thirolf. As it happens, lasers are not the only way of producing the excited state of the thorium nucleus. It also occurs when radioactive nuclei decay into thorium-229. “So we start with the grandparents and great-grandparents of thorium, as it were.”
ISOLDE is forging new paths
These ancestors are called francium-229 and radium-229. As neither are found readily in nature, they have to be manufactured synthetically. Currently, there are very few places in the world that are capable of doing this. One of them is the ISOLDE laboratory at the European Organization for Nuclear Research (CERN) in Geneva, which has made possible the old dream of the alchemists – of transforming one element into another.
To accomplish this, scientists bombard uranium nuclei with protons accelerated to extremely fast speeds, thereby producing various new nuclei – including francium and radium. These elements decay rapidly into the radioactive parent nucleus of thorium-229: actinium-229.
Kraemer, Thirolf, and their international colleagues embedded this elaborately manufactured actinium into special crystals, where the actinium decays into thorium in an excited state. When the thorium jumps back into its ground state, it emits the light particles whose frequency is so crucial for the development of the nuclear clock. Demonstrating this is no trivial task, however.
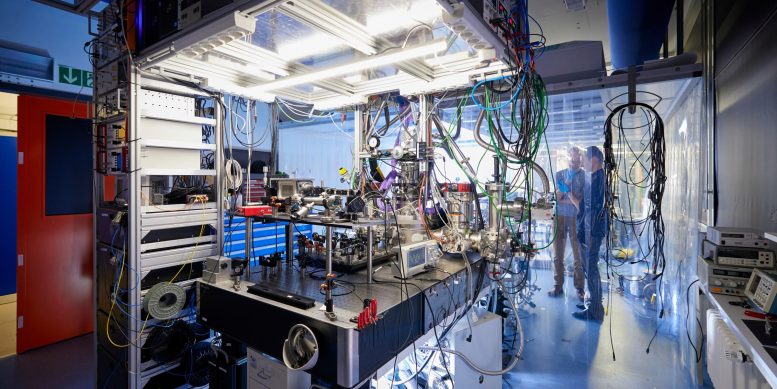
The next experiments to study the nucleus of thorium-229 in the laboratory of Sandro Kraemer and Peter Thirolf will start in the summer. Credit: Stephan Höck / LMU
“If the nuclei do not sit in exactly the right place in the crystal, we’ve got no chance,” says Kraemer. “The electrons in the environment absorb the energy and nothing that we can measure makes it outside.”
Previous attempts that inserted uranium into the crystal lattice instead of actinium fell at this hurdle. “When uranium-233 decays into thorium-229, a recoil is produced that wreaks havoc in the crystal,” explains Thirolf. The decay of actinium into thorium, by contrast, causes much less damage, which is why the researchers chose this laborious path for the new study in collaboration with CERN.
The hard work and patience have paid off: With their new method, the team was able to determine the energy of the state transition very precisely. They also demonstrated that a nuclear clock based on thorium embedded in a crystal is feasible. Such solid-state-based clocks would have the advantage over other approaches in that they would yield measurement results much more quickly because they work with a larger number of atomic nuclei.
A matter of time
“We now know the approximate wavelength we need,” says Thirolf. Building on the new findings to progressively narrow down the exact transition energy will be the next task. First, the researchers will create an excitation with a laser. And then they can keep homing in on the frequency with increasing accuracy with more precise lasers. So that this does not take too long, they do not use tweezers to find the needle in a haystack, so to speak, but a rake.
This ‘rake’ is called a “frequency comb” and was developed by Thirolf’s LMU colleague Professor Theodor Hänsch, who received the Nobel Prize in Physics in 2005 for the achievement. Scientists can use the comb to scan hundreds of thousands of wavelengths simultaneously until they find the right one.
Some challenges remain on the road to nuclear clocks. Scientists must understand the thorium isomer better, develop lasers, and work out theories. “But it’s worth sticking the course,” reckons Thirolf. “The project opens up such a plethora of new application possibilities in the long run that it’s worth all the experimental effort,” adds Kraemer.
These new possibilities encompass not only fundamental physics research but also practical applications. With a nuclear clock, scientists could detect the tiniest changes in the Earth’s gravitational field, such as occur when tectonic plates shift or ahead of volcanic eruptions. With the new successes, the prize is within arm’s reach. The first prototypes could be here in less than ten years. “We might even have them ready in time for the redefinition of the second in 2030,” the two physicists hope. They are referring to plans to come up with a new, more precise standard definition of a second, for which scientists will use state-of-the-art atomic clocks – and perhaps even the first nuclear clocks.
Reference: “Observation of the radiative decay of the 229Th nuclear clock isomer” by Sandro Kraemer, Janni Moens, Michail Athanasakis-Kaklamanakis, Silvia Bara, Kjeld Beeks, Premaditya Chhetri, Katerina Chrysalidis, Arno Claessens, Thomas E. Cocolios, João G. M. Correia, Hilde De Witte, Rafael Ferrer, Sarina Geldhof, Reinhard Heinke, Niyusha Hosseini, Mark Huyse, Ulli Köster, Yuri Kudryavtsev, Mustapha Laatiaoui, Razvan Lica, Goele Magchiels, Vladimir Manea, Clement Merckling, Lino M. C. Pereira, Sebastian Raeder, Thorsten Schumm, Simon Sels, Peter G. Thirolf, Shandirai Malven Tunhuma, Paul Van Den Bergh, Piet Van Duppen, André Vantomme, Matthias Verlinde, Renan Villarreal and Ulrich Wahl, 24 May 2023, Nature.
DOI: 10.1038/s41586-023-05894-z







The essence of time is the rotation of topological vortices, whose spin periods are both absolute and relative.
Look. Finish you vegetables before starting on the pudding. Where is my universal theory? I want my universal theory ! Give me my universal theory or I will hold my breath untill, .. well, untill I need to breathe. How do you like THEM apples?
Don’t try me, I’ll do it !!
There are atomic clocks installed at both the National Bureau of Standards at Boulder, Colorado, and at the Royal Observatory at Greenwich, England. Both are considered accurate to better than one-millionth of a second per year. However, the clock at Boulder ticks five microseconds faster per year than the one at Greenwich, England. Which one is correct? They both are! The one at Boulder is at an altitude of 5400 ft. above sea level. The one at Greenwich is only 80 ft. above sea level. The difference is caused by the fact that time is different due to the change in gravity. In 1971, J. C. Hafele and Richard Keating sent four cesium clocks around the world. The clocks on the eastward trip returned 59 nanoseconds behind the ones remaining at rest at the U.S. Naval Observatory. The clocks on the westward trip were 273 nanoseconds ahead. Accounting for the Earth’s rotation and other gravitational effects, this was precisely what Einstein’s formulas predicted.
So where the nuclear clock is located is going to matter. Since time is relative, who’s to say where it should be located?
Excellent!