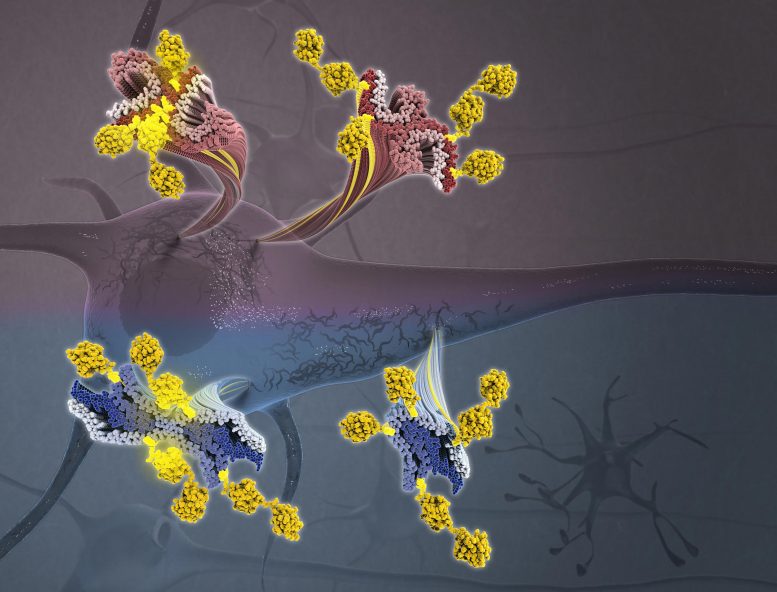
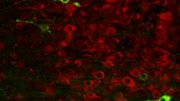
Tau aggregation into insoluble filaments is the defining pathological hallmark of tauopathies. Arakhamia et al. use cryo-electron microscopy and mass spectrometry to compare the structures of tau filaments from corticobasal degeneration and Alzheimer’s disease, identifying the importance of posttranslational modifications in mediating the structural diversity of tauopathy strains. Above: Tau filaments decorated with ubiquitin chains (yellow) from corticobasal degeneration (blue/navy) and Alzheimer’s disease (red/purple) emanating from a neuron. Credit: Veronica Falconieri Hays and Anthony Fitzpatrick/Columbia University’s Zuckerman Institute
Columbia-led team harnesses two powerful technologies to identify promising targets for diagnosing and treating neurodegenerative diseases.
The protein tau has long been implicated in Alzheimer’s and a host of other debilitating brain diseases. But scientists have struggled to understand exactly how tau converts from its normal, functional form into a misfolded, harmful one. Now, researchers at Columbia University’s Zuckerman Institute and Mayo Clinic in Florida have used cutting-edge technologies to see tau in unprecedented detail. By analyzing brain tissue from patients, this research team has revealed that modifications to the tau protein may influence the different ways it can misfold in a person’s brain cells. These differences are closely linked to the type of neurodegenerative disease that will develop — and how quickly that disease will spread throughout the brain.
The study, published today (February 6, 2020) in Cell, employed two complementary techniques to map the structure of tau and decipher the effects of additional molecules, called post-translational modifications (PTMs), on its surface. These new structural insights could accelerate the fight against neurodegenerative diseases, by helping researchers identify new biomarkers that detect these disorders before symptoms arise and design new drugs that target specific PTMs, preventing the onset of disease before it wreaks havoc on the brain.
“Tau has long been a protein of significant interest due to its prevalence in disease,” said Anthony Fitzpatrick, Ph.D., a Principal Investigator at Columbia’s Mortimer B. Zuckerman Mind Brain Behavior Institute who led the study. “In today’s publication, we lay out compelling evidence that PTMs play an important structural role in tauopathies, the collection of neurodegenerative diseases characterized by toxic buildup of misfolded tau.”
No two tauopathies are exactly alike. Each affects different parts of the brain — even different cell types — which can lead to different symptoms. Alzheimer’s, for example, arises in the hippocampus, and so affects memory. Chronic traumatic encephalopathy, a disorder most often seen in survivors of traumatic brain injury, can lead to problems with movement, memory or emotion, depending on which areas of the brain are affected.
Scientists have used traditional imaging techniques to find clues to how tangles of tau, comprised of individual fibers, or filaments, are implicated in these diseases. But painting a complete picture has proven difficult.
“The brains of patients with neurodegenerative diseases are easy to identify: entire sections have been eaten away, replaced by large clumps and tangles of misfolded proteins like tau,” said Tamta Arakhamia, an undergraduate at Columbia’s School of General Studies, a research assistant in the Fitzpatrick lab and the paper’s co-first author. “However, tau filaments are 10,000 times thinner than the width of a human hair, making them extraordinarily difficult to study in detail.”
To address this challenge, Dr. Fitzpatrick recently pioneered the use of cryo-electron microscopy, or cryo-EM, to visualize individual tau filaments from diseased human brain tissue. Cryo-EM is a Nobel Prize-winning technology developed, in part, by researchers at Columbia University. Cryo-EM image samples using a beam of electrons and has proven indispensable for investigations into extremely small biological structures. Using cryo-EM, Dr. Fitzpatrick’s team has reconstructed the structures of tau filaments, providing new insights into how they form, grow, and spread throughout the brain.
For all its ability to provide highly detailed snapshots of proteins, cryo-EM has limits. To overcome these limits, Dr. Fitzpatrick and his team to paired it with a second technology: mass spectrometry.
“Cryo-EM does not provide a complete picture because it cannot fully recognize the microscopic PTMs on tau’s surface,” said Christina Lee, an undergraduate student at Columbia College, a research assistant in the Fitzpatrick lab, and the paper’s co-first author. “But mass spectrometry can pinpoint the chemical composition of PTMs on the surface of tau.”
Working with co-corresponding author Leonard Petrucelli, Ph.D., Ralph B. and Ruth K. Abrams Professor of Neuroscience at Mayo Clinic in Florida, and Nicholas Seyfried, Ph.D., professor of biochemistry at Emory University School of Medicine, the researchers used cryo-EM and mass spectrometry to analyze the brain tissue from patients diagnosed with two tauopathies: Alzheimer’s disease and corticobasal degeneration, or CBD. CBD is a rare but extremely aggressive tauopathy, affecting only one in every 10,000 people. Unlike Alzheimer’s, which is thought to arise due to a number of factors including tau, CBD is primarily associated with misbehaving tau proteins.
“Studying a primary tauopathy like CBD helps us to figure out how tau becomes toxic to brain cells,” said Dr. Petrucelli. “We hope to extrapolate that knowledge to secondary tauopathies, such as Alzheimer’s disease.”
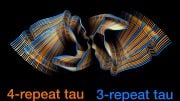
The scientists’ analysis of brain tissue samples revealed several key insights. Most notably, the researchers found that cross-talk between PTMs on the surface of tau influences the structure of the tau filaments, contributing to differences in tau filaments observed across the various tauopathies — and even variations from patient to patient.
“Collectively, these results suggest that PTMs may not only be serving as markers on the proteins’ surface, but are actually influencing the behavior of tau,” said Dr. Fitzpatrick, who is also an assistant professor of biochemistry and molecular biophysics at Columbia’s Vagelos College of Physicians and Surgeons.
Moving forward, Dr. Fitzpatrick and his team plan to expand this work to other tauopathies. Today’s findings on Alzheimer’s and CBD hold immense promise for the field, particularly in the development of new disease models — such as lab-grown organoids, or mini-brains — that may serve to accurately recapitulate what is actually happening in the brains of patients.
“Our findings will inspire new approaches for developing diagnostic tools and designing drugs, such as targeting PTM vulnerabilities to slow disease progression,” said Dr. Fitzpatrick, who is also a member of Columbia’s Taub Institute for Research on Alzheimer’s Disease and the Aging Brain. “Neurodegenerative diseases are among the most complex and distressing class of illnesses, but through our work and that of our colleagues and collaborators, we are building a roadmap toward successful diagnostics and therapeutics.”
Reference: “Posttranslational modifications mediate the structural diversity of tauopathy strains” by Tamta Arakhamia, Christina E. Lee, Yari Carlomagno, Duc M. Duong, Sean R. Kundinger, Kevin Wang, Dewight Williams, Michael DeTure, Dennis W. Dickson, Casey N. Cook, Nicholas T. Seyfried, Leonard Petrucelli and Anthony W.P. Fitzpatrick, 6 February 2020, Cell.
DOI: 10.1016/j.cell.2020.01.027
Additional contributors include Yari Carlomagno, Ph.D., Duc Duong, Sean Kundinger, Kevin Wang, Dewight Williams, Ph.D., Michael DeTure, Ph.D., Dennis Dickson, MD, and Casey Cook, Ph.D.
This research was supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke and National Institute on Aging (U01NS110438, RF1AG056151, R35NS097273, U01NS110438-02, P01NS084974, P01NS099114, R01NS088689, RF1AG062077-01, RF1 AG062171-01, U54NS100693, R01AG053960, R01AG061800, U01AG046161, U01AG061357, S10RR23057, S10OD018111, U24GM116792), NYSTAR and the NIH (GM103310), the National Science Foundation (MRI Grant 1531991, DBI-1338135, DMR-1548924), the Simons Foundation (349247), the Mayo Clinic Foundation, the Association for Frontotemporal Degeneration, the Dana Foundation, and the Cure Alzheimer’s Fund.
The authors report no financial or other conflicts of interest.
Columbia University’s Mortimer B. Zuckerman Mind Brain Behavior Institute brings together a group of world-class scientists and scholars to pursue the most urgent and exciting challenge of our time: understanding the brain and mind. A deeper understanding of the brain promises to transform human health and society. From effective treatments for disorders like Alzheimer’s, Parkinson’s, depression and autism to advances in fields as fundamental as computer science, economics, law, the arts and social policy, the potential for humanity is staggering.




Be the first to comment on "Toxic Protein Tau Exposed – Linked to Alzheimer’s and Neurodegenerative Diseases"