
Researchers at Cedars-Sinai have conducted the most comprehensive analysis of retinal changes in Alzheimer’s disease patients and their correlation with brain and cognitive changes. Published in the journal Acta Neuropathologica, this study is crucial for understanding the intricate effects of Alzheimer’s on the retina, particularly in the early stages of cognitive impairment. This understanding is vital for developing more effective treatments to halt the disease’s progression.
Cedars-Sinai Investigators Map Changes to the Retina That Correspond to Brain Changes in the Early Stage, Opening a Path to Earlier Diagnosis
An analysis led by Cedars-Sinai investigators is an important step toward understanding the complex effects of Alzheimer’s disease on the retina, a layer of tissue at the back of the eye.
Cedars-Sinai investigators have produced the most extensive analysis to date of changes in the retina—a layer of tissue at the back of the eye where visual information originates—and how those retinal changes correspond to brain and cognitive changes in Alzheimer’s disease patients.
Their analysis, published in the peer-reviewed journal Acta Neuropathologica, is an important step toward understanding the complex effects of Alzheimer’s disease on the retina, especially at the earliest stages of cognitive impairment. Experts believe this understanding is key for the development of more effective treatments that could prevent progression of the disease.
More than 3 million Americans are diagnosed with Alzheimer’s disease each year. The disease progressively destroys memory and cognitive ability. Currently, there is no single diagnostic test that can definitively diagnose a patient with Alzheimer’s disease, and the newest treatments only slow–don’t stop—progression.
“Our study is the first to provide in-depth analyses of the protein profiles and the molecular, cellular, and structural effects of Alzheimer’s disease in the human retina and how they correspond with changes in the brain and cognitive function,” said Maya Koronyo-Hamaoui, PhD, professor of Neurosurgery, Neurology, and Biomedical Sciences at Cedars-Sinai and senior author of the study. “These findings may eventually lead to the development of imaging techniques that allow us to diagnose Alzheimer’s disease earlier and more accurately and monitor its progression noninvasively by looking through the eye.”
“The retina, a developmental extension of the brain, offers an unparalleled opportunity for affordable, noninvasive monitoring of the central nervous system,” said Yosef Koronyo, MSc, research associate in the Cedars-Sinai Department of Neurosurgery and first author of the study. “And with the help of our collaborators, we discovered the accumulation of highly toxic proteins in the retinas of patients with Alzheimer’s disease and mild cognitive impairment, causing severe degeneration of cells.”
Investigators looked at retinal and brain tissue samples collected over 14 years from 86 human donors—the largest group of retinal samples from human patients with Alzheimer’s disease and mild cognitive impairment thus far studied. They compared samples from donors with normal cognitive function to those with mild cognitive impairment at the earliest stages of Alzheimer’s disease, and those with later-stage Alzheimer’s disease dementia.
The investigators explored the physical features of the retinas of these patients, measuring and mapping markers of inflammation and functional cell loss, and analyzed the proteins present in retinal and brain tissues.
Here is what investigators found in the retinas of patients with mild cognitive impairment and Alzheimer’s disease:
- An overabundance of a protein called amyloid beta 42, which in the brains of Alzheimer’s disease patients clumps together to form plaques that disrupt brain function
- Accumulation of amyloid beta protein in ganglion cells, the cells that bridge visual input from the retina to the optic nerve
- Higher numbers of astrocytes and immune cells, called microglia, tightly surrounding amyloid beta plaques
- As many as 80% fewer microglial cells clearing amyloid beta proteins from the retina and brain
- Specific molecules and biological pathways responsible for inflammation, and cell and tissue death
“These changes in the retina correlated with changes in parts of the brain called the entorhinal and temporal cortices, a hub for memory, navigation, and the perception of time,” said Koronyo.
Retinal changes also correlated with the pathological stage of Alzheimer’s disease (called Braak stage) and patients’ cognitive status. And they were found even in patients who appeared cognitively normal or very mildly impaired, marking them as a possible early predictor of later cognitive decline.
“These findings give us a deeper understanding of the effects of Alzheimer’s disease on the retina,” said Keith L. Black, MD, chair of the Department of Neurosurgery and the Ruth and Lawrence Harvey Chair in Neuroscience at Cedars-Sinai and a co-author of the study. “Because these changes correspond with changes in the brain and can be detected in the earliest stages of impairment, they may lead us to new diagnostics for Alzheimer’s disease and a means to evaluate new forms of treatment.”
Reference: “Retinal pathological features and proteome signatures of Alzheimer’s disease” by Yosef Koronyo, Altan Rentsendorj, Nazanin Mirzaei, Giovanna C. Regis, Julia Sheyn, Haoshen Shi, Ernesto Barron, Galen Cook-Wiens, Anthony R. Rodriguez, Rodrigo Medeiros, Joao A. Paulo, Veer B. Gupta, Andrei A. Kramerov, Alexander V. Ljubimov, Jennifer E. Van Eyk, Stuart L. Graham, Vivek K. Gupta, John M. Ringman, David R. Hinton, Carol A. Miller, Keith L. Black, Antonino Cattaneo, Giovanni Meli, Mehdi Mirzaei, Dieu-Trang Fuchs and Maya Koronyo-Hamaoui, 11 February 2023, Acta Neuropathologica.
DOI: 10.1007/s00401-023-02548-2
Funding: This work was supported by the National Institutes of Health (NIH) National Institute on Aging grant numbers R01AG056478, R01AG055865, AG056478-04S1, and R01AG062007; NIH National Institute of General Medical Sciences grant number R01 GM132129; NIH National Eye Institute grant number R01EY013431; the Haim Saban, Maurice Marciano, and Tom Gordon private foundations; Australian National Health and Medical Research Council grant numbers GNT1128436, GNT1129192, and GNT1139469; the Clem Jones Centre for Ageing Dementia Research; the National Health and Medical Research Council; the Petersen Foundation; the Australian Government’s National Collaborative Research Infrastructure Scheme; Fondo Ordinario Enti of Italian CNR; and Macquarie University.




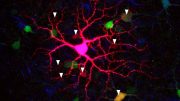



Re: “…Specific molecules and biological pathways responsible for inflammation, and cell and tissue death…”
I hope my email to the corresponding author effectively informs them of the likelyhood (e.g., “experience-based” medicine) of uric acid being that mysterious (to them) factor.