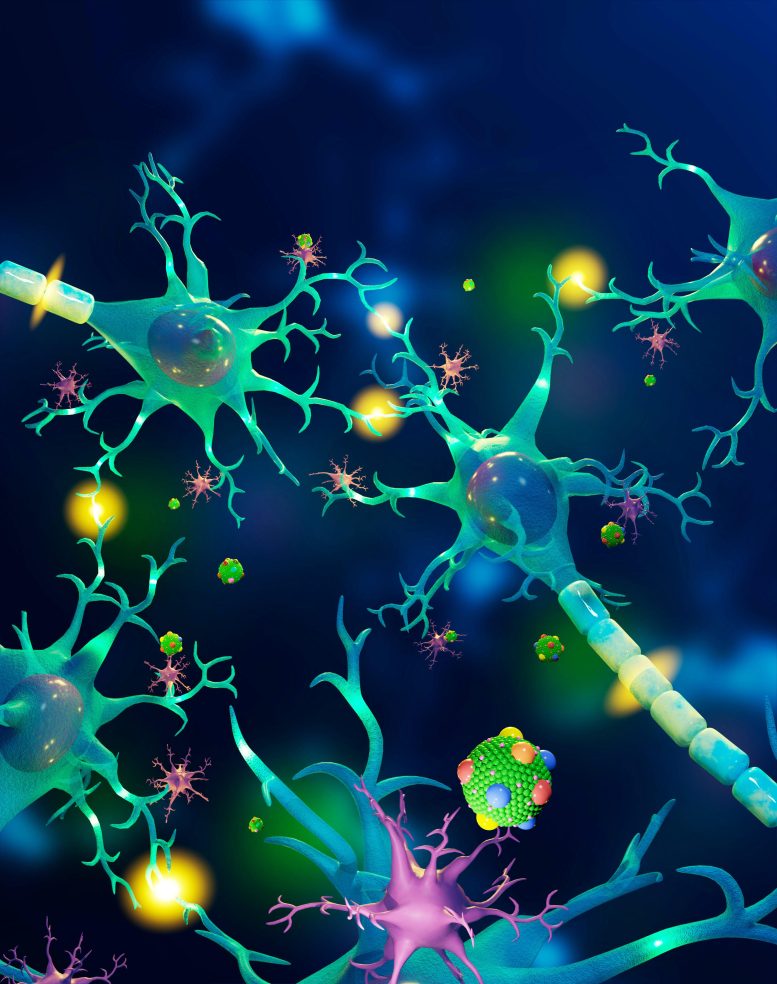
A spherical lipoprotein article with proteins on the surface is visible in the lower part of the image in this artist’s rendition that includes neurons (light-green cells), synapses (connections highlighted in yellow), and microglia (in purple). Credit: Mike Perkins | Pacific Northwest National Laboratory
New research provides a fresh glimpse of APOE, scores of new molecular players in the central nervous system.
Researchers have developed a technique to identify key fat-filled particles known as lipoproteins within the central nervous system, opening a new view into the workings of the brain.
The study revealed that these particles, molecular cousins of the well-known HDL, or “good cholesterol” particles in our bloodstream, are much more diverse than previously thought. The team identified over 300 distinct proteins linked with these particles, a significant increase from the previously known 16, that fall into at least 10 different families.
These particles are rich in proteins that affect wound healing, the immune response, and the creation and nurturing of brain cells called neurons which are important for cognitive function.
The most common protein on the particles is apolipoprotein E, better known as APOE. Of the three commonly studied forms of APOE, the form known as APOE4 puts people at higher risk of Alzheimer’s disease. One copy of the APOE4 gene makes a person approximately four times as likely to develop dementia; a person with two copies is about 12 times more vulnerable.
The results were recently published in the journal Science Advances. The leader of the study is John Melchior, a protein biochemist at the Department of Energy’s Pacific Northwest National Laboratory. Melchior is a leader in lipoprotein research—and a carrier of two copies of the APOE4 gene, adding a personal incentive to his work to understand the protein’s action in the nervous system.
“We’ve known for a long time that in the nervous system, APOE is the primary protein on these particles calling the shots. But we don’t know much more beyond that. Our technology opens the door to learning more,” said Melchior.
“What the heck is APOE4 doing? That’s the big question. Why does one form translate to less risk for dementia while a slightly different form confers significant risk? Our technology brings us one step toward more answers,” he added.
APOE: Bringing fats and proteins together
Lipoproteins are best known for their work in the circulatory system, where they transport fat and cholesterol. It’s easy for scientists to detect HDL and LDL, known to many as “good cholesterol” and “bad cholesterol,” in the bloodstream because the molecules there are plentiful.
However, lipoproteins in the nervous system are much scanter, present at less than 1 percent of the concentration in blood. Their actions, even their presence, in the nervous system have been a mystery. Scientists know that these balls of fat and protein wrapped together to travel in the bloodstream and carry out all sorts of important functions, like shuttling cholesterol and nutrients.
On the particles in the central nervous system, APOE reigns supreme, serving as a scaffold to hold lipids and other proteins together. It also transports these nutrient-rich lipids and collections of molecular collaborators—groupings of proteins on its surface—throughout the nervous system to perform their tasks. The proteins are specialized tools that can do things like repair cells, turn genes on or off, or regulate amyloid-beta processing, a well-known molecule related to the development of dementia. The work suggests that APOE may bring these tools together on different particles to deliver them where needed.
But something is more likely to go wrong in people with one or two copies of APOE4, leading to dementia. Scientists don’t know what.
Scientists suspect APOE4 of playing a role in other neurologic conditions such as Parkinson’s and Huntington’s diseases, multiple sclerosis, amyotrophic lateral sclerosis, and even traumatic brain injury.
Because lipoproteins are less common in the nervous system, researchers either need an impossibly large amount of the cerebrospinal fluid to study them—or scientists develop a new way to detect the rare molecules. That’s what Melchior’s team did, creating a new fluorescent technology to tag lipoproteins in spinal fluid.
The team studied just one-third of a milliliter of spinal fluid—much less fluid than in a raindrop—and discovered 303 different proteins across the particle families using mass spectrometry. Most had never been detected on these particles in the nervous system before.
A solid start and next steps: lipoproteins and neurological diseases
“Now comes the fun part,” said Melchior. “We want to open up our technology to clinicians to learn more about what’s happening in Alzheimer’s disease and possibly other conditions like multiple sclerosis and Parkinson’s disease.
“There are existing cerebrospinal fluid samples sitting in freezers, and we have a new way to analyze them. We’d love to work together with other research teams to investigate them. The sooner we can start profiling lipoproteins in these conditions, the sooner we can understand more about their role in disease pathology and identify targets for treatments,” added Melchior, who holds joint appointments at Oregon Health & Science University and the University of Cincinnati.
First author of the paper is PNNL scientist Nathaniel Merrill who did much of the computational analysis of the data. Merrill designed computational tools to help sort out extraordinarily complicated datasets. This includes which proteins are most likely to be found together on different particle populations and what processes those proteins control in the central nervous system.
Reference: “Human cerebrospinal fluid contains diverse lipoprotein subspecies enriched in proteins implicated in central nervous system health” by Nathaniel J. Merrill, W. Sean Davidson, Yi He, Ivo Díaz Ludovico, Snigdha Sarkar, Madelyn R. Berger, Jason E. McDermott, Linda J. Van Eldik, Donna M. Wilcock, Matthew E. Monroe, Jennifer E. Kyle, Kimberley D. Bruce, Jay W. Heinecke, Tomas Vaisar, Jacob Raber, Joseph F. Quinn and John T. Melchior, 30 August 2023, Science Advances.
DOI: 10.1126/sciadv.adi5571
The study brought together investigators from the University of Cincinnati, OHSU, University of Washington, University of Kentucky, University of Colorado, VA Portland Healthcare System as well as PNNL. In addition to Melchior and Merrill, PNNL authors include Ivo Díaz Ludovico, Snigdha Sarkar, Madelyn Berger, Jason McDermott, Matthew Monroe and Jennifer Kyle.
The work was done as part of the Pacific Northwest Biomedical Innovation Co-Laboratory, or PMedIC, where PNNL and OHSU scientists and physicians work together to bring basic science and clinical experience together to explore disease and develop innovative therapies. The work was funded by the National Institutes of Health (1R03AG070480, P01HL128203, R01AG079217, P30AG066518) and by the OHSU School of Medicine.
The early support for the project from NIH was crucial, said W. Sean Davidson of the University of Cincinnati, a coauthor and an expert on lipoproteins in the circulatory system.
“We took advantage of the Notice of Special Interest program, which allows labs that are funded in other areas—in our case, cardiovascular disease—to translate new technologies toward Alzheimer’s disease. This made it possible to turn a crazy idea into an exciting new way to analyze lipoproteins in the brain,” said Davidson.




Be the first to comment on "Unraveling the Secrets of Alzheimer’s – Researchers Create a New Window on Leading Genetic Cause"