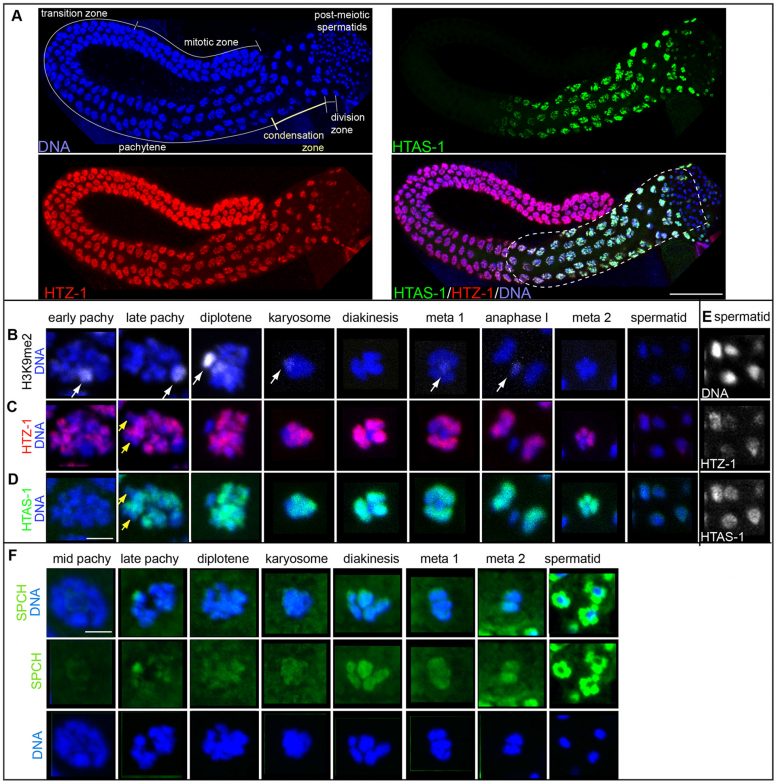
Immunolocalization of isolated and fixed C. elegans male gonads. A) DNA stained with DAPI (blue), HTAS-1 (green), HTZ-1 (red), merged image of HTAS-1 (green), HTZ-1 (red), and DAPI (blue). The scale bar represents 50 µm. B–E) Immunostaining of individual nuclei from late stages of sperm formation (as found in the proximal end of the gonad marked with the dotted line in A). The scale bar represents 2 µm and applies to all panels in B–F. B) Histone H3 dimethylated at lysine 9 (H3K9me2), which marks the X chromosome (white arrows). C) HTZ-1. D) HTAS-1. Yellow arrows mark regions of under-representation of HTAS-1 and HTZ-1 that are not the X chromosome. E) HTZ-1 and HTAS-1 are detectable immediately after meiotic divisions as sperm chromatin condenses. Contrast-adjusted black and white images of DNA, HTZ-1, and HTAS-1 staining of the early spermatid nuclei shown in panels C and D that show HTZ-1 and HTAS-1 are detectable. See Figure S2 for contrast-adjusted images of later spermatid nuclei. F) SPCH proteins (SPCH-1, 2, 3) (green) are detectable on DNA as sperm DNA condenses for meiotic divisions. Levels of SPCH proteins increase dramatically after meiosis, particularly around spermatid DNA. Credit: Samson M, et al., PLoS Genet 10(10): e1004588. doi:10.1371/journal.pgen.1004588
New research from San Francisco State University sheds light on the multilayered process of how a sperm and egg pass along information needed for successful reproduction, revealing that embryos receive parent-specific layers of information.
The information that interprets the genetic code in a new embryo differs depending on whether it comes from the father or mother, researchers at San Francisco State University have found.
The research, detailed in an article published in the journal PLOS Genetics, sheds light on the multilayered process of how a sperm and egg pass along information needed for successful reproduction. Though one layer is the DNA code that is transferred, the new study identifies information not encoded by DNA, a so-called “epigenetic” layer of information that helps the cell interpret the genetic code.
Scientists have known these “epigenetic marks,” which influence the developmental plan of new embryos, are created by biologically modifying the proteins, called histones, that are responsible for tightly coiling DNA inside cells. But the new study shows how distinctive the marks from a sperm cell are from the information coming from an oocyte, or egg cell.
“We were able to document an array of marks from dad that are different than what is passed over from mom,” said Diana Chu, an associate professor of biology at SF State. “This research opens up new avenues for scientists to investigate. What is the role of these different marks? Why are they different?”
Chu and her colleagues at SF State and the Scripps Research Institute examined sperm and embryos of the C. elegans worm to identify which histone marks, which include histone variants and modifications, were unique to sperm and track them during the formation of new sperm cells and new embryos. They found that the number of histone modifications present within sperm is 2.4 times less than the number present within embryos, indicating a widespread erasure of epigenetic marks when new sperm cells are formed. But the erasure is incomplete: Researchers identified one histone variant and six histone modifications — and there are likely many more — that are retained in sperm and ultimately passed on to a new embryo, potentially helping it develop properly.
“If the DNA is the book, these variants and modifications are the bookmarks,” Chu said. “They can help cells read the book.”
The discovery adds another dimension for scientists to explore when studying the complex process of how genetic information is passed on from parents to children. Such research has major health implications, Chu added, as developmental and behavioral disorders that may not manifest themselves until years or even decades into an individual’s life can originate in the early stages of embryonic development.
“These various levels of genetic and epigenetic information work in combination,” she said. “If you don’t understand all of them, you can’t understand the complexity of how cells divide properly and how they create a human being who can function at many different levels for a long period of time.”
Further research will identify additional epigenetic marks that are unique to sperm, as well as study more closely the role and features of a specific histone variant, termed HTAS-1.
“The specification and global reprogramming of histone epigenetic marks during gamete formation and early embryo development in C. elegans” by Mark Samson, Margaret M. Jow, Catherine C.L. Wong, Colin Fitzpatrick, Aaron Aslanian, Israel Saucedo, Rodrigo Estrada, Takashi Ito, Robin Park, John R. Yates III, and Diana S. Chu was published in the October 9 edition of PLOS Genetics. The research was funded through several grants from the National Science Foundation.
Reference: “The Specification and Global Reprogramming of Histone Epigenetic Marks during Gamete Formation and Early Embryo Development in C. elegans” by Mark Samson, Margaret M. Jow, Catherine C. L. Wong, Colin Fitzpatrick, Aaron Aslanian, Israel Saucedo, Rodrigo Estrada, Takashi Ito, Sung-kyu Robin Park, John R. Yates III and Diana S. Chu, 9 October 2014, PLOS Genetics.
DOI: 10.1371/journal.pgen.1004588








Be the first to comment on "Researchers Show Embryos Receive Parent-Specific Layers of Information"