
Japanese researchers have discovered ice 0, a new type of ice that forms near the surface of water, potentially redefining scientific understanding and influencing technology and climate studies. (Artist’s concept). Credit: SciTechDaily.com
Researchers from Japan have discovered a new form of ice called ice 0, which can seed the formation of ice crystals in supercooled water. Their study reveals that ice nucleation can occur near the surface of water droplets due to ice 0-like structures, resolving a longstanding debate. These findings have significant implications for various fields, including climate studies and food sciences, by enhancing our understanding of ice formation.
Ice is far more complex than most people realize, with science identifying over 20 different varieties formed under various combinations of pressure and temperature. The type we use to chill our drinks, known as ice I, is one of the few forms that occur naturally on Earth. Recently, researchers from Japan discovered another type: ice 0, an unusual form of ice that can initiate the formation of ice crystals in supercooled water.
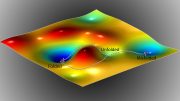
The formation of ice near the surface of liquid water can start from tiny crystal precursors with a structure similar to a rare type of ice, known as ice 0. In a study recently published in Nature Communications, researchers from the Social Cooperation Research Department “Frost Protection Science,” at the Institute of Industrial Science, The University of Tokyo showed that these ice 0-like structures can cause a water droplet to freeze near its surface rather than at its core. This discovery resolves a longstanding puzzle and could help redefine our understanding of how ice forms.
Ice Nucleation Process
Crystallization of ice, known as ice nucleation, usually happens heterogeneously, or in other words, at a solid surface. This is normally expected to happen at the surface of the water’s container, where liquid meets solid. However, this new research shows that ice crystallization can also occur just below the water’s surface, where it meets the air. Here, the ice nucleates around small precursors with the same characteristic ring-shaped structure as ice 0.
“Simulations have shown that a water droplet is more likely to crystallize near the free surface under isothermal conditions,” says lead author of the study Gang Sun. “This resolves a longstanding debate about whether crystallization occurs more readily on the surface or internally.”

Researchers from the Institute of Industrial Science, The University of Tokyo have found that ice starts forming near the surface of water via structures similar to a rare, recently discovered type of ice, which helps us understand ice formation better. Credit: Institute of Industrial Science, The University of Tokyo
Ice 0 Precursors
Ice 0 precursors have a structure very similar to supercooled water, allowing water molecules to crystallize more readily from it, without needing to directly form themselves into the structure of regular ice. The tiny ice 0 precursors are formed spontaneously, as a result of negative pressure effects caused by the surface tension of water. Once crystallization begins from these precursors, structures similar to ice 0 quickly rearrange themselves into the more familiar ice I.
Senior author, Hajime Tanaka stresses the wide-ranging implications of this study, noting that, “The findings regarding the mechanism of surface crystallization of water are expected to contribute significantly to various fields, including climate studies and food sciences, where water crystallization plays a critical role.”
A more detailed understanding of ice and how it forms can give invaluable insight into a variety of areas of study. This work may have particular importance in meteorology, for example, where ice formation via ice 0-like precursors may have a much more noticeable effect in small water droplets like those found in clouds. Understanding ice can have benefits in technology too, from food sciences to air conditioning.
Reference: “Surface-induced water crystallisation driven by precursors formed in negative pressure regions” by Gang Sun, and Hajime Tanaka, 26 July 2024, Nature Communications.
DOI: 10.1038/s41467-024-50188-1






Japanese researchers have discovered ice 0, a new type of ice that forms near the surface of water, potentially redefining scientific understanding and influencing technology and climate studies.
VERY GOOD!
According to the topological vortex gravitational field theory, the interaction and balance via the topological vortex gravitational field, it is difficult for two water molecules, two hydrogen atoms, two oxygen atoms, or even two photons to be absolutely identical, let alone ice.
Scientific research guided by correct theories can help humanity avoid detours, failures, and pomposity. Please witness the exemplary collaboration between theoretical physicists and experimentalists (https://scitechdaily.com/microscope-spacecrafts-most-precise-test-of-key-component-of-the-theory-of-general-relativity/#comment-854286). Contemporary physics has always lived in a self righteous children’s story world. Whose values have been overturned by such a comical and ridiculous reality?
In the superposition, deflection, and entanglement of topological vortices, the change of symmetry is always the Symmetry-Readjusted, not the Symmetry-Broken. The physical phenomena observed in scientific research are not precisely symmetrical. That’s because it’s difficult for us to observe the entirety of the world that is interactions and interconnections. Being in the world of spin wave interactions of topological vorties, any asymmetry is a local manifestation of overall symmetry adjustment.
Misguided by the pseudo-scientific theory of Physical Review Letters (PRL), many researchers do not consider the similarities and differences between geometric shapes and physical reality in physics research, but indulge in imagination. Although scientific research can be imagined, it cannot be done recklessly. Perhaps one day, humans will truly understand the immense power of symmetry.
Bollox!