
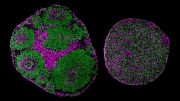
Brain organoids in a laboratory dish. Credit: UC San Diego Health Sciences
Nine-month-old brains-in-a-dish and the brains of premature newborn babies generate similar electrical patterns, as captured by electroencephalogram (EEG) — the first time such brain activity has been achieved in a cell-based laboratory model.
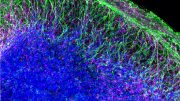
Brain organoids — also called mini-brains — are 3D cellular models that represent aspects of the human brain in the laboratory. Brain organoids help researchers track human development, unravel the molecular events that lead to disease and test new treatments. They aren’t perfect replicas, of course. Brain organoids do not replicate cognitive function, but researchers can check how an organoid’s physical structure or gene expression changes over time or as a result of a virus or drug.
University of California San Diego researchers have now taken brain organoids one step further, achieving an unprecedented level of neural network activity — electrical impulses that can be recorded by multi-electrode arrays. Using data from babies born up to three-and-a-half months premature, the team developed an algorithm to predict their age based on EEG patterns. The algorithm then read lab-grown brain organoids the same way and assigned them an age.
The electrical impulse pattern for nine-month-old brain organoids revealed similar features to those of a premature infant who had reached full-term (40 weeks gestation).
These new optimized brain organoids, described in the August 29, 2019 issue of Cell Stem Cell, may make it possible for researchers to study mental illnesses that aren’t caused by or result in overt physiological changes, but instead involve disturbances in brain cell network activity, such as autism or epilepsy. For many of these conditions, there are no relevant laboratory or animal models.
“We couldn’t believe it at first — we thought our electrodes were malfunctioning,” said co-senior author Alysson R. Muotri, Ph.D., professor of pediatrics and cellular and molecular medicine at UC San Diego School of Medicine. “Because the data were so striking, I think many people were kind of skeptical about it, and understandably so.” Muotri led the study with Bradley Voytek, Ph.D., associate professor of cognitive science in the UC San Diego Division of Social Sciences.
Brain organoid construction begins with a perhaps surprising source: an adult skin sample. In the lab, researchers convert the skin cells into induced pluripotent stem cells (iPSCs). Like most stem cells, with the right cocktail of molecular factors, iPSCs can be directed to specialize into any cell type. In this case, they become brain cells — different types of neurons and glia, for example.
At UC San Diego, brain organoids have been used to produce the first direct experimental proof that the Brazilian Zika virus can cause severe birth defects and to repurpose existing HIV drugs for a rare, inherited neurological disorder. Muotri and team also recently sent their brain organoids to the International Space Station to test microgravity’s effect on brain development — and maybe prospects for human life beyond Earth.
In the latest study, Muotri and colleagues optimized every step of brain organoid construction. For example, they started from single cells, rather than the clumps of cells used in most protocols. They also tweaked the precise timing and concentration of factors added to prompt brain cell organization. There wasn’t a single secret ingredient or innovation, he said, but rather several improvements over time.
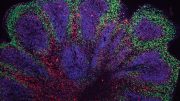
The optimization paid off in terms of cellular diversity and cellular network activity. For example, the team detected a particular primate-specific neuron, called a cortical GABAergic neuron, that had never before been generated in a lab dish. According to Muotri, these cells are important players in the sophistication of neural networks.
To measure cellular network activity, the researchers grew their newly optimized brain organoids on multi-electrode arrays. The electrodes capture and record electrical impulses, which appear as patterns of waves and spikes in an EEG read-out. With the new protocol, the brain organoids went from producing 3,000 spikes per minute to 300,000 spikes per minute.
In humans, oscillations change with age, as brain cell connectivity develops. Newborn baby brains tend to have periods of rest (no waves) between spikes of electrical activity. Those quiet periods get shorter and shorter as the brain develops. In time, brain activity becomes constant, though levels vary. These brain oscillation patterns often correlate with human cognition and disease states.
Muotri and team compared their brain organoid electrical patterns to a publicly available dataset of 567 EEG recordings from 39 babies born prematurely, between 24 and 38 weeks gestation, and for several weeks after birth. From their initial days to nine months, the brain organoids produced similar levels of electric activity, following a similar pattern: less quiet time, more frequent electrical impulses.
A detailed overview of a study conducted by Alysson Muotri’s lab at the UC San Diego Stem Cell Program which found complex network signaling developing in human cortical organoids that appear to recapitulate fetal brain development, offering an in-vitro model to study functional development of human neuronal networks.
Muotri said he is often asked about the ethical implications of this work, with questions like: “Are we getting too close to re-creating the human brain?” These brain organoids dramatically differ from human brains in many ways, he explained. For example, they are several times smaller than an adult human brain. They do not have hemispheres or blood vessels. And they are not surrounded by protective skulls or connected to other tissues.
“They are far from being functionally equivalent to a full cortex, even in a baby,” said Muotri, who is also director of the UC San Diego Stem Cell Program and a member of the Sanford Consortium for Regenerative Medicine. “In fact, we don’t yet have a way to even measure consciousness or sentience.”
Muotri’s brain organoids can live for years in the lab, but their activity plateaus at nine months. He said a number of reasons might apply, including the lack of blood vessels or the need for additional neurons to continue maturing.
The better brain organoids can replicate the human brain in the lab, Muotri said, the fewer researchers will need to rely on animal models and fetal tissue to better understand and treat human disease.
“Our work doesn’t yet replace the need for human fetal brain tissue for research, but it’s very attractive as a potential alternative,” he said.
Co-authors include: Cleber A. Trujillo, Richard Gao, Priscilla D. Negraes, Jing Gu, Justin Buchanan, Sebastian Preissl, Allen Wang, Wei Wu, Gabriel G. Haddad, Isaac A. Chaim, Alain Domissy, Matthieu Vandenberghe, UC San Diego; Anna Devor, UC San Diego and Harvard Medical School; Gene W. Yeo, UC San Diego, Agency for Science, Technology and Research, and National University of Singapore.
This research was funded, in part, by National Institutes of Health (grants R01MH108528, R01MH094753, R01MH109885, R01MH100175, R56MH109587, K12GM06852, U19MH1073671), National Science Foundation (grant 1736028), California Institute for Regenerative Medicine (CIRM grants DISC1-08825, DISC2-09649), Simons Foundation (SFARI grant 345469), Brain & Behavior Research Foundation (NARSAD Independent Investigator Grant), Sloan Research Fellowship, Whitehall Foundation (grant 2017-12-73), Natural Sciences and Engineering Research Council of Canada (NSERC PGS-D), UC San Diego Kavli Innovative Research Grant, Frontiers for Innovation Scholars Program and Katzin Prize.
Disclosure: Alysson Muotri is a co-founder and has equity interest in TISMOO, a company dedicated to genetic analysis and brain organoid modeling focusing on therapeutic applications customized for autism spectrum disorder and other neurological disorders with genetic origins. Gene Yeo is co-founder, member of the board of directors and scientific advisory board, equity holder and paid consultant for Locana and Eclipse BioInnovations. Yeo is also a visiting faculty at the National University of Singapore. The terms of these arrangements have been reviewed and approved by the University of California San Diego in accordance with its conflict of interest policies.
Reference: “Complex Oscillatory Waves Emerging from Cortical Organoids Model Early Human Brain Network Development” by Cleber A. Trujillo, Richard Gao, Priscilla D. Negraes, Jing Gu, Justin Buchanan, Sebastian Preissl, Allen Wang, Wei Wu, Gabriel G. Haddad, Isaac A. Chaim, Alain Domissy, Matthieu Vandenberghe, Anna Devor, Gene W. Yeo, Bradley Voytek and Alysson R. Muotri, 29 August 2019, Cell Stem Cell.
DOI: 10.1016/j.stem.2019.08.002





Be the first to comment on "AI Algorithm Can’t Distinguish These Lab Mini-Brains from Preemie Babies [Video]"