
A new study has discovered that crystal structures, crucial in materials science and technologies like semiconductors and solar panels, are not necessarily always regularly arranged. They found that the random stacking of hexagonal layers (RHCP), previously considered a transitional state, is likely stable and could provide new useful properties in polytypic materials like silicon carbide used in high-voltage electronics and body armor.
Previous beliefs have been overturned by the discovery of irregularly arranged structures.
For many, the word “crystals” conjures images of shimmering suncatchers that create a prism of rainbow colors or semi-transparent stones thought to possess healing abilities. But in the realm of science and engineering, crystals take on a more technical definition. They’re perceived as materials whose components – be it atoms, molecules, or nanoparticles –are arranged regularly in space. In other words, crystals are defined by the regular arrangement of their constituents. Familiar examples include diamonds, table salt, and sugar cubes.

Sangwoo Lee. Credit: Rensselaer Polytechnic Institute
Contrary to this widely accepted definition, a recent study led by Sangwoo Lee, an associate professor in the Department of Chemical and Biological Engineering at Rensselaer Polytechnic Institute, has unveiled an intriguing aspect of crystal structures revealing that the arrangement of components within crystals isn’t always necessarily regular.
The discovery advances the field of materials science and has unrealized implications for the materials used for semiconductors, solar panels, and electric vehicle technologies.
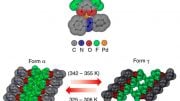
One of the most common and important classes of crystal structures is the close-packed structures of regular spheres constructed by stacking layers of spheres in a honeycomb arrangement. There are many ways to stack the layers to construct close-packed structures, and how nature selects specific stacking is an important question in materials and physics research. In the close-packing construction, there is a very unusual structure with irregularly spaced constituents known as the random stacking of two-dimensional hexagonal layers (RHCP). This structure was first observed from cobalt metal in 1942, but it has been regarded as a transitional and energetically unpreferred state.
Lee’s research group collected X-ray scattering data from soft model nanoparticles made of polymers and realized that the scattering data contains important results about RHCP but is very complicated. Then, Patrick Underhill, professor in Rensselaer’s Department of Chemical and Biological Engineering, enabled the analysis of the scattering data using the supercomputer system, Artificial Intelligence Multiprocessing Optimized System (AiMOS), at the Center for Computational Innovations.
“What we found is that the RHCP structure is, very likely, a stable structure, and this is the reason that RHCP has been widely observed in many materials and naturally occurring crystal systems,” said Lee. “This finding challenges the classical definition of crystals.”
The study provides insights into the phenomenon known as polytypism, which enables the formation of RHCP and other close-packed structures. A representative material with polytypism is silicon carbide, widely used for high-voltage electronics in electric vehicles and as hard materials for body armor. Lee’s team’s findings indicate that those polytypic materials may have continuous structural transitions, including the non-classical random arrangements with new useful properties.
“The problem of how soft particles pack seems straightforward, but even the most basic questions are challenging to answer,” said Kevin Dorfman of the University of Minnesota-Twin Cities, who is unaffiliated with this research. “This paper provides compelling evidence for a continuous transition between face-centered cubic (FCC) and hexagonal close-packed (HCP) lattices, which implies a stable random hexagonal close-packed phase between them and, thus, makes an important breakthrough in materials science.”
“I am particularly pleased with this discovery, which shows the power of advanced computation to make an important breakthrough in materials science by decoding the molecular level structures in soft materials,” said Shekhar Garde, dean of Rensselaer’s School of Engineering. “Lee and Underhill’s work at Rensselaer also promises to open up opportunities for many technological applications for these new materials.”
Reference: “Continuous transition of colloidal crystals through stable random orders” by Juhong Ahn, Liwen Chen, Patrick T. Underhill, Guillaume Freychet, Mikhail Zhernenkovc and Sangwoo Lee, 14 April 2023, Soft Matter.
DOI: 10.1039/D3SM00199G
Lee and Underhill were joined in research by Rensselaer’s Juhong Ahn, Liwen Chen of the University of Shanghai for Science and Technology, and Guillaume Freychet and Mikhail Zhernenkov of Brookhaven National Laboratory.








X-ray diffraction analysis has shown that there are similar transitions in minerals such as magnesium containing calcites. So-called Hi-mag calcite. These are biological minerals formed by foraminifera in the oceans.