
Researchers from Münster University have developed a new photocatalytic process to activate water using triaryl phosphines, facilitating easier hydrogen production from water. This innovative approach uses light energy to drive the reaction and shows potential for exploring new chemical processes using hydrogen atoms in synthesis.
Photocatalytic process enables water to be activated.
Hydrogen is often touted as a future energy solution, especially when generated through environmentally friendly methods. Beyond its energy potential, hydrogen plays a crucial role in producing active ingredients and various essential compounds. To generate hydrogen, water (H2O) can be transformed into hydrogen gas (H2) through a sequence of chemical reactions.
However, as water molecules are very stable, splitting them into hydrogen and oxygen presents a big challenge to chemists. For it to succeed at all, the water first has to be activated using a catalyst – then it reacts more easily.
A team of researchers led by Prof. Armido Studer at the Institute of Organic Chemistry at Münster University (Germany) has developed a photocatalytic process in which water, under mild reaction conditions, is activated through triaryl phosphines and not, as in most other processes, through transition metal complexes.
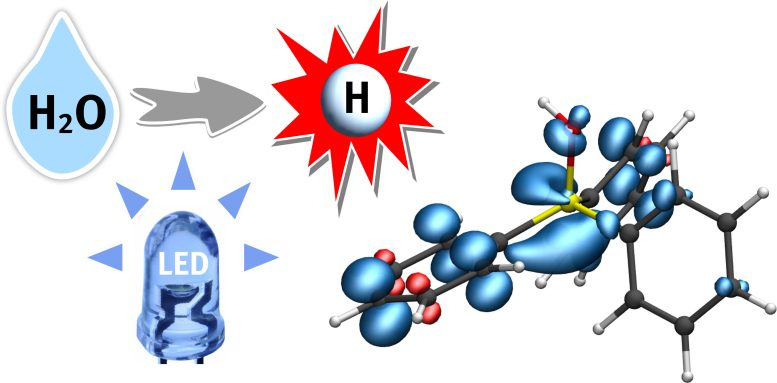
A hydrogen atom (H) from water (H2O) is transferred to a phosphine-water radical cation under the supply of light energy (LED). This important radical intermediate can further transfer the hydrogen atom (white) to the substrate. The blue regions indicate the electron spin distribution. Credit: Christian Mück-Lichtenfeld
This strategy, which was recently published in the journal Nature, will open a new door in the highly active field of research relating to radical chemistry, says the team. Radicals are, as a rule, highly reactive intermediates. The team uses a special intermediate – a phosphine-water radical cation – as activated water, from which hydrogen atoms from H2O can be easily split off and transferred to a further substrate.
The reaction is driven by light energy. “Our system,” says Armido Studer, “offers an ideal platform for investigating unresearched chemical processes that use the hydrogen atom as a reagent in synthesis.”
Dr. Christian Mück-Lichtenfeld, who analyzed the activated water complexes using theoretical methods, says, “The hydrogen-oxygen bond in this intermediate is extraordinarily weak, making it possible to transfer a hydrogen atom to various compounds.” Dr. Jingjing Zhang, who carried out the experimental work, adds: “The hydrogen atoms of the activated water can be transferred to alkenes and arenes under very mild conditions, in so-called hydrogenation reactions.”
Hydrogenation reactions are enormously important in pharmaceutical research, in the agrochemical industry, and in materials sciences.
Reference: “Photocatalytic phosphine-mediated water activation for radical hydrogenation” by Jingjing Zhang, Christian Mück-Lichtenfeld and Armido Studer, 28 June 2023, Nature.
DOI: 10.1038/s41586-023-06141-1
The study was funded by Alexander von Humboldt-Stiftung.








That’s nice, but for other than the pharmaceutical or agricultural trade, I see little point to it.
“… water, under mild reaction conditions, is activated through triaryl phosphines …”
Is the production of triaryl phosphines ‘environmentally friendly?’ If water is decomposed on a large scale, there will be dissolved salts left behind that will need to be disposed of. Can that be done in an ‘environmentally friendly’ manner?
As NASA has discovered, there is no such thing as a free launch. The way the process is described, it sounds like it takes little energy to break the hydrogen-oxygen bonds. Since I don’t believe in perpetual motion machines, I suspect the energy comes from the production of the triaryl phosphines. If that is so, then the required energy is just shifted from water to producing the catalyst.
Will they soon be making Ice-9?
What if you pressurize the water
Why would you suggest that? Are there any fundamental chemical properties that you can cite that would lead you to believe that it would be possible to break the strong chemical bonds more easily?
What if you centrifugaly spin the water and use a hydrolyzer, try on both sides of the hemisphere
What about the plasmoid generator??
Might make more sense to derive H2 from hydrocarbons instead of our water. Although the combustion of H2 and O2 produces water again so it depends how they plan to use the H2 to generate power.
The fundamentals here are the area of panels needed to drive the hydrogen extraction and where does the oxygen go? I assume it bubbles off and the triaryl phosphines has to be ‘regenerated’ somewhere to extract the hydrogen.
No idea of the economics which are critical. I see all sorts of articles on electrolysis efficiency but not much that mentions that the energy of the hydrogen is about 40 KWhr so you need electricity at about 2 and a half cents to match NG decomposition, even at 100% efficiency. That is possible with ‘excess generation during wind or solar peaks but it really needs to be discussed more.