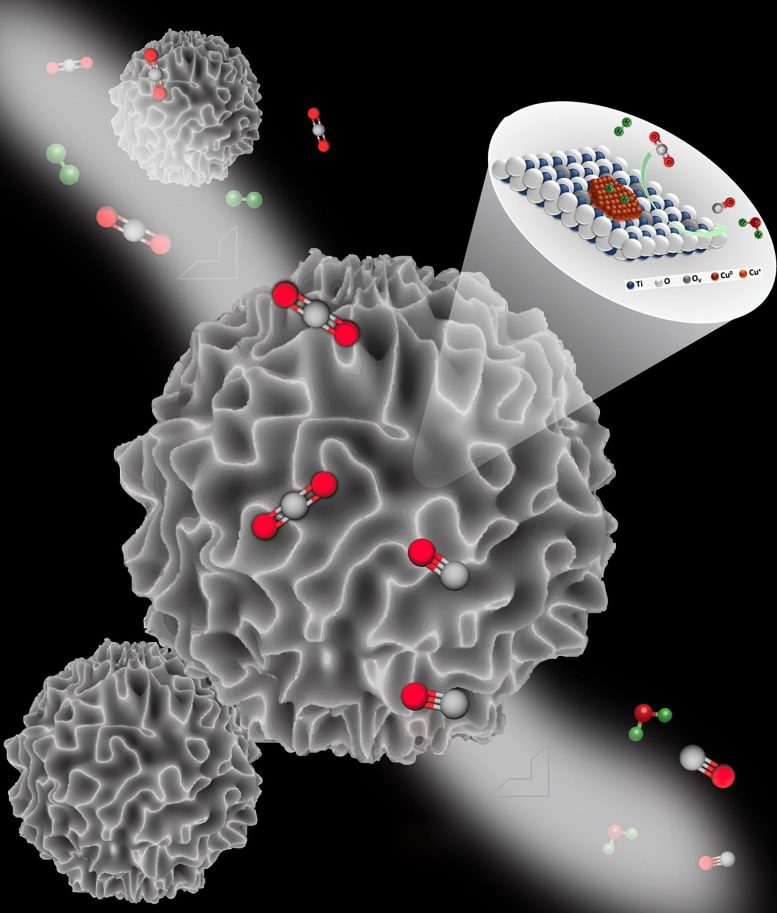
“Defects Tune the Strong Metal–Support Interactions,” a unique approach to design CO2 reduction nanocatalyst with excellent efficiency and stability. Credit: Mr. Rajesh Belgamwar and Prof. Vivek Polshettiwar
A team of researchers has developed a highly efficient and stable copper-based catalyst (DFNS/TiO2-Cu) for converting CO2 to CO, with a CO productivity of 5350 mmol g−1 h−1 and 99.8% selectivity. The catalyst remained stable for over 200 hours and in-situ studies highlighted the importance of defect sites in tuning strong metal-support interactions.
The heavy use of fossil fuels for driving industrial processes and human activities has resulted in increasingly excessive emissions of anthropogenic CO2 into our atmosphere, surpassing the 400 ppm level. This exceedingly high concentration of atmospheric CO2 has led to a series of negative consequences for our planet’s climate system. However, CO2 can be a strategic carbon resource for synthesizing valued chemicals and fuels. There have been numerous reports of noble metal catalysts, but their application was limited due to their moderate catalytic performance and high cost. In the non-noble metal catalyst family, Cu-based catalysts are among the most versatile, with good potential in many industrial processes. Unfortunately, the low Tammann temperature of copper and the resulting surface migration causes nanoparticles to sinter during the reaction, limiting their activity and long-term stability.
In this work, a Team of researchers led by Prof. Vivek Polshettiwar at Tata Institute of Fundamental Research (TIFR), Mumbai, asked the question, how to improve the catalytic activity and stability of Cu-catalyst using the concept of strong metal support interactions (SMSI) and defect sites cooperativity?
They reported a catalyst with active copper sites loaded on titanium oxide-coated dendritic fibrous nanosilica (DFNS/TiO2-Cu) for CO2 to CO conversion. The fibrous morphology and high surface area of DFNS/TiO2 allowed better dispersion and high loading of Cu NPs active sites. This catalyst showed excellent catalytic performance for CO2 reduction with CO productivity of 5350 mmol g−1 h−1 (i.e., 53506 mmol gCu−1 h−1), superior to all copper-based thermal catalysts. Notably, DFNS/TiO2-Cu10 showed 99.8% selectivity towards CO and was stable for at least 200 hours. The defect-controlled strong metal-support interactions between Cu and TiO2 kept the copper nanoparticles firmly anchored on the surface of the support and imparted excellent catalyst stability.
The EELS studies, in-situ diffuse reflectance infrared Fourier transform spectroscopy, H2-temperature-programmed reduction, density functional theory calculations, and long-term stability indicated that there was a strong interaction between copper sites and the Ti3+ sites, which ensured good stability and dispersion of the active copper sites. In-situ studies provided insights into the role of defect sites (Ti3+ and O-vacancies) in tuning SMSI. In-situ time-resolved Fourier transform infrared indicated that CO2 did not directly dissociate to form CO, while the in-situ Raman and in-situ UV-DRS study demonstrated that the intensity of the oxygen vacancies and Ti3+ centers gradually decreased after introducing CO2 gas into the reactor chamber and progressively increased when exposed to hydrogen. This indicated that CO2 to CO conversion followed a redox pathway assisted by hydrogen.
Excellent catalytic performance of DFNS/TiO2-Cu and in-situ mechanistic studies indicated the potential of defects in tuning the strong metal-support interactions. This approach may lead to the design of catalytic systems using various active sites and defective supports.
Reference: “Defects Tune the Strong Metal–Support Interactions in Copper Supported on Defected Titanium Dioxide Catalysts for CO2 Reduction” by Rajesh Belgamwar, Rishi Verma, Tisita Das, Sudip Chakraborty, Pradip Sarawade, and Vivek Polshettiwar, 5 April 2023, Journal of the American Chemical Society.
DOI: 10.1021/jacs.3c01336
Funding: Department of Atomic Energy (DAE), Government of India, Mission Innovation India, Department of Science and Technology, Government of India







Why convert CO2 to CO?
Did I miss a reference in the article concerning a use for CO in pharmacology or elsewhere?
“This EXCEEDINGLY high concentration of atmospheric CO2 has led to a series of negative consequences for our planet’s climate system.”
Just how was it determined that the atmospheric CO2 is “exceedingly high?” It has been at least an order of magnitude higher in the past when life was abundant and diverse. While changes are taking place that humans may subjectively find troubling, like rising sea levels, NASA has documented that vegetation is expanding on Earth in what has been characterized as “greening.” Surely a greater abundance and diversity of life is a desirable condition. If that proposition is accepted, then how can something contributing to the condition be excessive?
I think that scientists should stick to objective facts, like the numbers resulting from measurements, and not dabble in ‘bio-geo-poetry’ with subjective, ambiguous adverbs.
Here I thought C0 (Carbon MONOXIDE) was way more dangerous than C02 seeing I have a Carbon Monoxide detector in my house so I don’t drop dead from a furnace leak. Yes, let’s convert plant friendly C02 into an even more deadly gas instead. Brilliant. Just BRILLIANT.