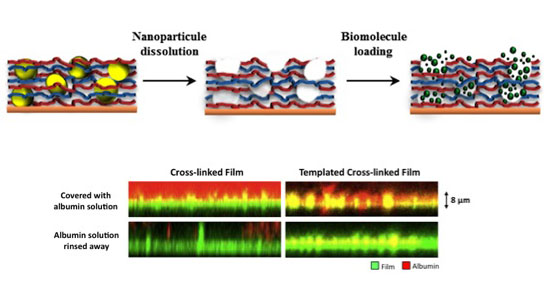
(Top) The researchers’ new templating approach. (Bottom) Laser scanning confocal microscopy images show cross-linked films without (left) and with (right) nanoparticle templating. Credit: SEAS; Yale University News
In a newly published study, a team of researchers describes their new approach towards creating polymeric thin film biomaterials that achieve mechanical rigidity in conjunction with bioactivity.
Biomaterials are ubiquitous in modern medicine, from prosthetic limbs to device coatings, and hold promise for future applications like scaffolding for tissue regeneration. Biomaterial design presents a key challenge, however: applications often require materials to be both mechanically rigid (to promote strong cell adhesion) and bioactive (i.e. able to communicate specific cues to contacting cells). To date, achieving one of these features usually requires sacrificing the other.
“Increasing film rigidity through chemical crosslinking often suppresses film bioactivity, whether by inhibiting the mobility or accessibility of embedded biomolecules or by chemically altering them,” says Connie Wu, a chemical engineering undergraduate student and lead author of the paper. “Films that retain both structural integrity and bioactivity would prove useful in applications such as scaffold coatings in tissue engineering or drug delivery.”
In a new paper in Advanced Functional Materials, Wu and graduate student Seyma Aslan, working with others from the groups of Paul Van Tassel of Yale SEAS and Emmanuel Pauthe of the University of Cergy-Pontoise (France), present an idea that appears to address this challenge: a thin polymer film biomaterial offering both high rigidity and bioactivity. The approach involves forming the film in the presence of sacrificial nanoparticle templates.
The film is first rigidified through a chemical cross-linking procedure (similar to the way glue hardens); then, the template species are selectively dissolved, leaving behind a pore structure somewhat like that of Swiss cheese (but with much smaller holes!). These pores can then be filled with proteins able to communicate with cells, rendering the film bioactive.
The new approach – part of an ongoing effort toward polymeric thin film biomaterials – achieves mechanical rigidity in conjunction with bioactivity, and, notably, allows for embedding high quantities of biological species without regard to size (previous methods were limited to lower quantities of smaller drug or protein species).
“Porous polymer films would be ideal for applications in cell-based therapies, where materials of optimal rigidity and tailored bioactivity are needed to support cellular implants or transplants,” Van Tassel states, while adding “additional research is needed, though, to determine optimal combinations of embedded bioactive species.”
Reference: “Porous Nanofilm Biomaterials Via Templated Layer-by-Layer Assembly” by Connie Wu, Seyma Aslan, Adeline Gand, Joseph S. Wolenski, Emmanuel Pauthe Paul R. Van Tassel, 10 August 2012, Advanced Functional Materials.
DOI: 10.1002/adfm.201201042








Be the first to comment on "Designing Biomaterials Both High in Rigidity and Bioactivity"