Exeter scientists have discovered a simple, efficient way to recreate the early structure of the human embryo from stem cells in the laboratory. The new approach unlocks new ways of studying human fertility and reproduction.
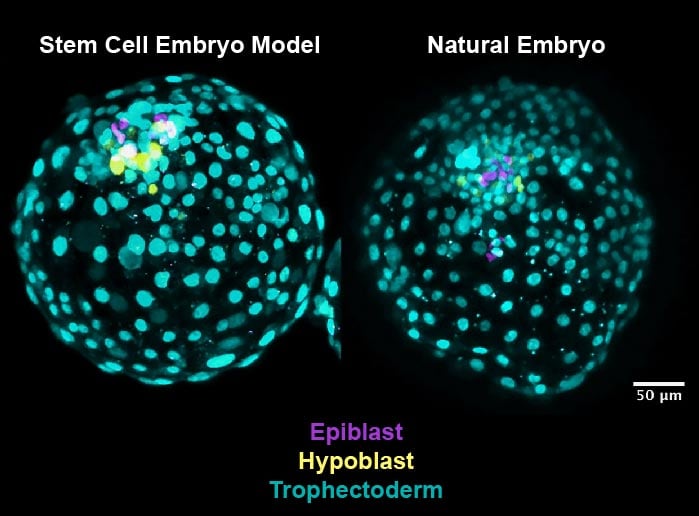
Stem cells have the ability to turn into different types of cells. Now, in research published in Cell Stem Cell and funded by the Medical Research Council, scientists at the University of Exeter’s Living Systems Institute, working with colleagues from the University of Cambridge, have developed a method to organize lab-grown stem cells into an accurate model of the first stage of human embryo development.
The ability to create artificial early human embryos could benefit research into infertility, by furthering understanding of how embryos develop, and the conditions needed to avoid miscarriage and other complications. The embryo models can also be used to test conditions that may improve the development of embryos in assisted conception procedures such as IVF.
The new discovery comes after the team found that a human stem cell was able to generate the founding elements of a blastocyst – the very early formation of an embryo after a fertilized egg divides. Professor Austin Smith, Director of the University of Exeter’s Living Systems Institute, said: “Finding that stem cells can create all the elements of an early embryo is a revelation. The stem cells come from a fully-formed blastocyst, yet they are able to recreate exactly the same whole embryo structure. This is quite remarkable and unlocks exciting possibilities for learning about the human embryo.”
The research has the potential to significantly advance understanding. Few human embryos are available for study, so until now, scientists have largely focussed on animal research, particularly mice, despite the fact that their reproductive systems differ significantly from humans. Around one in seven couples in the UK has difficulty conceiving.
In the research, the team arranged the stem cells into clusters and briefly introduced two molecules known to influence how cells behave in early development. They found that 80 percent of the clusters organized themselves after 3 days into structures that look remarkably like the blastocyst stage of an embryo – a ball of around 200 cells that forms from the fertilized egg after 6 days. The team went on to show that the artificial embryos have the same active genes as the natural embryo.
The study was directed by Dr. Ge Guo, of the University of Exeter’s Living Systems Institute, said: “Our new technique provides for the first time a reliable system to study early development in humans without using embryos. This shouldn’t be seen as a move towards producing babies in a laboratory, but rather as an important research tool that could benefit IVF and infertility studies.”
The next stage for the researchers is to understand how to develop the artificial embryos a few days further to study the critical period when an embryo would implant into the womb, which is when many embryos fail to develop properly.
Reference: “Naive stem cell blastocyst model captures human embryo lineage segregation” by Ayaka Yanagida, Daniel Spindlow, Jennifer Nichols, Anish Dattani, Austin Smith and Ge Guo, 5 May 2021, Cell Stem Cell.
DOI: 10.1016/j.stem.2021.04.031





Be the first to comment on "Early Human Embryo Structure Created With Stem Cells in Advance for Fertility Research"