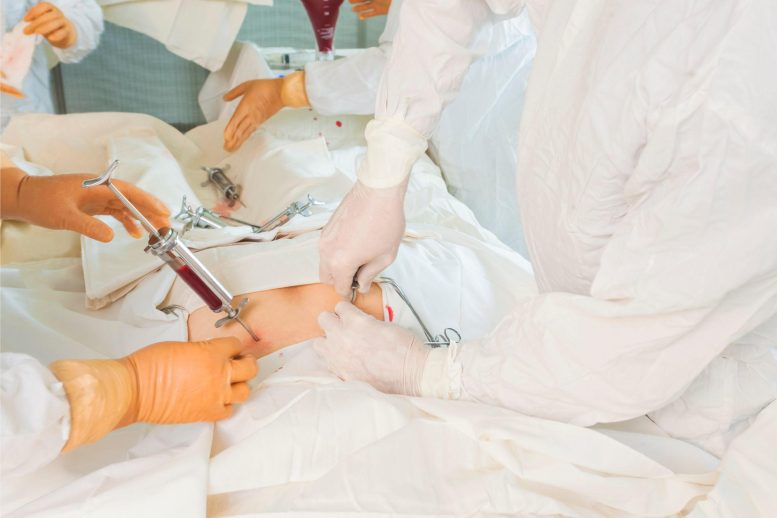
A bone marrow transplant, also known as a stem cell transplant, is a medical procedure in which a patient’s damaged or diseased bone marrow is replaced with healthy cells from a donor. The procedure is used to treat blood-related cancers such as leukemia and lymphoma, as well as other disorders such as sickle cell anemia and thalassemia.
The new drug has been shown to be faster and has fewer negative side effects compared to the current standard treatment.
A team of researchers at the Tisch Cancer Institute at Mount Sinai has developed a treatment that is more effective and safer than the current standard of care for a severe and potentially fatal side effect of bone marrow transplants in cancer patients. The results of a phase 2 clinical trial were presented at the annual meeting of the American Society of Hematology (ASH) in December.
The trial included adolescents and adults and found that a drug that suppresses the immune system in patients with graft-versus-host disease (GvHD) was safer than the current standard treatment, steroids. GvHD is a side effect experienced by patients who have received bone marrow transplants from donors to treat blood cancers. The study used a blood test created at Mount Sinai to identify patients with GvHD who would benefit most from the new treatment.
“Steroids are known to cause numerous complications in bone marrow transplant recipients who require treatment for GvHD such as serious infections, bone and muscle damage, poor sleep, and poor quality of life,” said Aaron Etra, MD, Assistant Professor of Medicine (Hematology and Medical Oncology) at The Tisch Cancer Institute, who presented the study at the ASH meeting.
“A steroid-free treatment for GvHD would be an important advance in improving transplant outcomes, but it’s not likely to be the best choice for all patients. Being able to use GvHD biomarkers to personalize treatment intensity was key to the success of this trial,” added John Levine, MD, Professor of Medicine (Hematology and Medical Oncology) at The Tisch Cancer Institute, and co-senior author for this research. Alexandra Capellini, a Mount Sinai medical student, was a co-first author.
GvHD occurs when the cells from a donor attack the healthy organs of the recipient. It results in the release of certain tissue proteins into the bloodstream; these proteins can be used as biomarkers to quantify the severity of the tissue damage. Patients with low levels of these biomarkers tend to respond well to treatment in general, but before now there was no way to identify them. A research laboratory at Mount Sinai was able to quantify the GvHD biomarkers in blood samples obtained from patients within 30 hours, which made it feasible to study this treatment in patients who need to start treatment quickly.
Results from the trial were compared to a matched control group of patients who were treated with steroids. This research found that a short course of itacitinib, a JAK1 inhibitor that can tamp down the immune system, produced very high response rates that occurred faster than treatment with steroids. Responses to itacitinib were as durable as steroids and long-term outcomes were equally good.
Both itacitinib and steroids were effective in 86 percent of patients. The one-year survival rate for itacitinib was 88 percent compared to 80 percent for steroids. Patients treated with itacitinib also had significantly fewer severe infections, likely a result of dramatically less exposure to systemic steroids.
“This was the first time anyone was able to use real-time GvHD biomarkers to identify low-risk patients whose treatment could be de-escalated,” said James L Ferrara, MD, Professor of Medicine (Hematology and Medical Oncology) at The Tisch Cancer Institute, and co-senior author for this research. “Mount Sinai developed this biomarker approach, which allowed us to present this new and meaningful finding for patients and their quality of life.”
Reference: 64th ASH Annual Meeting & Exposition
The study was funded by Incyte. Trial sites included MD Anderson Cancer Center, City of Hope, Ohio State University, Mass General, Mayo Clinic, Vanderbilt University, Emory University, Fred Hutchinson Cancer Research Center, University of Pennsylvania, Columbia University, University of Hamburg, University of Regensburg, and the University of Erlangen.


Be the first to comment on "Faster and Safer: A New Treatment for Bone Marrow Transplant Side Effects"