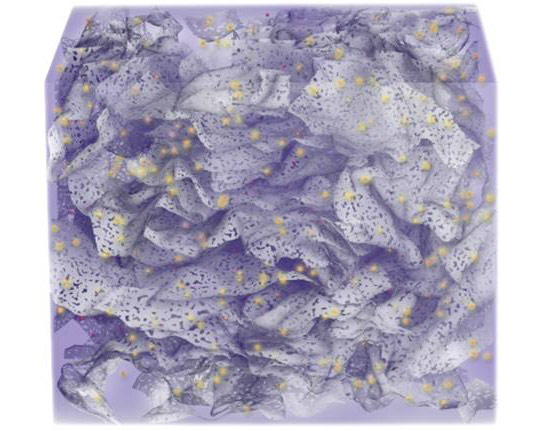
The initial solvated 3D hierarchical porous structure of holey graphene framework. Credit: UCLA CNSI
Scientists at UCLA have developed a new graphene material that bridges gap between traditional capacitors and batteries, boosting the energy density of electrochemical capacitors and putting them on a par with lead acid batteries.
Researchers at the California NanoSystems Institute (CNSI) at UCLA have set the stage for a watershed in mobile energy storage by using a special graphene material to significantly boost the energy density of electrochemical capacitors, putting them on a par with lead acid batteries.
The material, called a holey graphene framework, has a three-dimensional, perforated structure characterized by tiny holes; it not only increases energy density (the amount of energy stored and ready for use) but allows electrochemical capacitors to maintain their high power density (the amount of power per unit of mass or volume), according to Xiangfeng Duan, a UCLA professor of chemistry and biochemistry who led the research.
Electrochemical capacitors, also known as ECs or supercapacitors, are an important technology for the future of energy storage and mobile power supplies, but they have been limited by low energy density. Compared with traditional batteries, ECs typically have superior power density and cycle life — the number of complete charge–discharge cycles an energy source can support before it decreases to 80 percent of its original capacity and is considered “worn out.” But they have had energy density of at least one order of magnitude below batteries.
Because the main component of an EC is its electrode material, which is responsible for the EC’s overall performance, recent research has focused on efficient new materials that are able to increase energy density without sacrificing power density or cycle life. A high-performance EC electrode must have high electrical conductivity, a high ion-accessible surface area, a high ionic transport rate, and high electrochemical stability.
Current state-of-the-art ECs generally use porous activated carbon electrodes with energy densities much lower than lead acid batteries — 4 to 5 watt hours per kilogram vs. 25 to 35 watt hours per kilogram (5 to 7 watt hours per liter vs. 50 to 90 watt hours per liter).
In their study, published in the journal Nature Communications, the CNSI researchers led by Duan used a highly interconnected 3D holey graphene framework as the electrode material to create an EC with unprecedented performance. The electrode demonstrates superior electrical conductivity, exceptional mechanical flexibility, and unique hierarchical porosity, ensuring the efficient transport of electrons and ions and enabling the highest gravimetric energy densities of 127 watt hours per kilogram and volumetric energy density of 90 watt hours per liter.
Furthermore, the team has shown that a fully packaged EC exhibits unparalleled energy densities of 35 watt hours per kilogram (49 watt hours per liter) — about five to 10 times higher than current commercial supercapacitors and on a par with acid batteries.
“The holey graphene EC bridges the energy density gap between traditional capacitors and batteries, yet with vastly higher power density,” Duan said. “It creates exciting opportunities for mobile power supplies for many applications from cell phones to electric vehicles.”
Reference: “Holey graphene frameworks for highly efficient capacitive energy storage” by Yuxi Xu, Zhaoyang Lin, Xing Zhong, Xiaoqing Huang, Nathan O. Weiss, Yu Huang and Xiangfeng Duan, 8 August 2014, Nature Communications.
DOI: 10.1038/ncomms5554







Be the first to comment on "Holey Graphene Framework Boosts the Energy Density of Electrochemical Capacitors"