
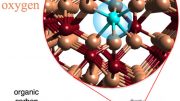
In this illustration, a “smart rust” nanoparticle attracts and traps estrogen molecules, which are represented by the floating objects. Credit: Dr. Dustin Vivod and Prof. Dr. Dirk Zahn, Computer Chemistry Center (CCC), Friedrich-Alexander-Universität Erlangen-Nürnberg
Researchers have developed “smart rust,” iron oxide nanoparticles that clean water by attracting pollutants such as oil, nano- and microplastics, glyphosate, and even estrogen hormones.
Pouring flecks of rust into water typically makes it dirtier. However, a groundbreaking development by researchers has led to the creation of “smart rust,” a type of iron oxide nanoparticle that can purify water. This smart rust has the unique ability to attract various pollutants, such as oil, nano- and microplastics, and the herbicide glyphosate, depending on the particles’ coating. What makes it even more efficient is its magnetic nature, which allows easy removal from water using a magnet, taking the pollutants along with it. Recently, the team has optimized these particles to capture estrogen hormones, which can be detrimental to aquatic life.
Presentation and Significance
The researchers presented their results at the fall meeting of the American Chemical Society (ACS). ACS Fall 2023 is a meeting that features about 12,000 presentations on a wide range of science topics.
The water in our oceans, lakes, and rivers can become polluted with a variety of contaminants, creating a need for a simple and cheap cleaning method. One team of researchers is designing magnetic nanoparticles that can target specific pollutants like estrogen hormones, which are carried into waterways by wastewater and may be harmful to aquatic life. The particles are made from iron oxide, which most of us know as rust, and researchers can modify the surface of the particles to grab onto various pollutants. Then, a magnet can pull the particles out of the water, along with any pollutants clinging to them. Credit: American Chemical Society
“Our ‘smart rust’ is cheap, nontoxic, and recyclable,” says Marcus Halik, Ph.D., the project’s principal investigator. “And we have demonstrated its use for all kinds of contaminants, showing the potential for this technique to improve water treatment dramatically.”
The Science Behind Smart Rust
For many years, Halik’s research team has been investigating environmentally friendly ways to remove pollutants from water. The base materials they use are iron oxide nanoparticles in a superparamagnetic form, which means they are drawn to magnets, but not to each other, so the particles don’t clump.
To make them “smart,” the team developed a technique to attach phosphonic acid molecules onto the nanometer-sized spheres. “After we add a layer of the molecules to the iron oxide cores, they look like hairs sticking out of these particles’ surfaces,” says Halik, who is at Friedrich-Alexander-Universität Erlangen-Nürnberg. Then, by changing what is bound to the other side of the phosphonic acids, the researchers can tune the properties of the nanoparticles’ surfaces to strongly adsorb different types of pollutants.
Early versions of smart rust trapped crude oil from water collected from the Mediterranean Sea and glyphosate from pond water collected near the researchers’ university. Additionally, the team demonstrated that smart rust could remove nano- and microplastics added to lab and river water samples.
Targeting Hormonal Pollutants
Thus far, the team has targeted pollutants present in mostly large amounts. Lukas Müller, a graduate student who’s presenting new work at the meeting, wanted to know if he could modify the rust nanoparticles to attract trace contaminants, such as hormones. When some of our body’s hormones are excreted, they are flushed into wastewater and eventually enter waterways. Natural and synthetic estrogens are one such group of hormones, and the main sources of these contaminants include waste from humans and livestock. The amounts of estrogens are very low in the environment, says Müller, so they are difficult to remove. Yet even these levels have been shown to affect the metabolism and reproduction of some plants and animals, although the effects of low levels of these compounds on humans over long periods aren’t fully known.
“I started with the most common estrogen, estradiol, and then four other derivatives that share similar molecular structures,” says Müller. Estrogen molecules have a bulky steroid body and parts with slight negative charges. To exploit both characteristics, he coated iron oxide nanoparticles with two sets of compounds: one that’s long and another that’s positively charged. The two molecules organized themselves on the nanoparticles’ surface, and the researchers hypothesize that together, they build many billions of “pockets” that draw in the estradiol and trap it in place.
Because these pockets are invisible to the naked eye, Müller has been using high-tech instruments to verify that these estrogen-trapping pockets exist. Preliminary results show efficient extraction of the hormones from lab samples, but the researchers need to look at additional experiments from solid-state nuclear magnetic resonance spectroscopy and small-angle neutron scattering to verify the pocket hypothesis. “We are trying to use different puzzle pieces to understand how the molecules actually assemble on the nanoparticles’ surface,” explains Müller.
Future Prospects
Looking ahead, the team intends to test these particles on real-world water samples and determine the number of times that they can be reused. Because each nanoparticle has a high surface area with lots of pockets, the researchers say that they should be able to remove estrogens from multiple water samples, thereby reducing the cost per cleaning. “By repeatedly recycling these particles, the material impact from this water treatment method could become very small,” concludes Halik.
Meeting: ACS Fall 2023
The researchers acknowledge support and funding from the German Research Foundation, the German Federal Environmental Foundation and Friedrich-Alexander-Universität Erlangen-Nürnberg.
Title
Smart rust to clean water from hormones
Abstract
Access to clean water is recognized as a human right by the United Nations. However, anthropogenic molecular pollutants, like hormones are present in our ground water and find their way into drinking water due to careless disposal and insufficient remediation. Already at the trace concentration level such compounds have been shown to have severe effects on aquatic flora and fauna, but also to us humans, especially children. Still consequences of long term exposure are often unknown. Therefore, it exists a big demand in affordable and efficient removal of such organic contaminants from water. Having this in mind, we are en route to develop a promising concept to solve this problem. Superparamagnetic iron oxide nanoparticles (SPIONs) are surface-functionalized with self-assembled monolayers (SAMs) composed of permanently binding phosphonic acid derivates to address certain interaction motifs of selected hormones (“smart rust”). Such particles attract the pollutants and can be easily remediated from water by an external magnetic field due to the magnetic moment of its cores. Basing on previous successful remediation of the herbicide glyphosate, micro- and nanoplastics as well as crude oil via single major interaction motifs (covalent binding – electrostatic interactions – hydrophobic interaction respectively), we pursue the next logical step. We establish the interaction of rationally designed mixed SAMs on SPIONs with dedicated trace organic pollutants, i. e. various estrogen derivates. Therefore, we envision sorbent systems that are not only thermodynamically attractive for the pollutants of choice by combination of multiple interaction motifs, but also present suitably-sized cavities in the mixed SAM. This approach benefits from synergy of experimental materials science and analytical chemistry to tailor hybrid nanoparticles.







A few years ago, I read that magnetite (FeO*Fe2O3) could be used to remove arsenic from water. Arsenic is a common problem in India, and sometimes encountered in the US.
This article is very helpful in understanding and creating new ways of nanotechnology uses.