
New research from CU Boulder indicates that by 2100, the acidity of Antarctica’s coastal waters may double, severely impacting marine life in the Southern Ocean. This increase in acidity, primarily due to CO2 emissions, threatens the entire ecosystem, including whales and penguins. Credit: SciTechDaily.com
Antarctica’s coastal waters may see a 100% increase in acidity by 2100, endangering marine life, according to a new study. Reducing CO2 emissions is crucial to mitigate this threat.
The acidity of Antarctica’s coastal waters could double by the end of the century, threatening whales, penguins, and hundreds of other species that inhabit the Southern Ocean, according to new CU Boulder research.
Scientists projected that by 2100, the upper 650 feet (200 meters) of the ocean—where much marine life resides—could see more than a 100% increase in acidity compared with 1990s levels. The paper was published on January 4 in the journal Nature Communications.
“The findings are critical for our understanding of the future evolution of marine ecosystem health,” said Nicole Lovenduski, the paper’s co-author and the interim director of CU Boulder’s Institute of Arctic and Alpine Research (INSTAAR).
Ocean Acidification: A Consequence of CO2 Emissions
The oceans play an important role as a buffer against climate change by absorbing nearly 30% of the CO2 emitted worldwide. But as more CO2 dissolves in the oceans, the seawater becomes more acidic. “Human-caused CO2 emissions are at the heart of ocean acidification,” said Cara Nissen, the paper’s first author and a research scientist at INSTAAR.
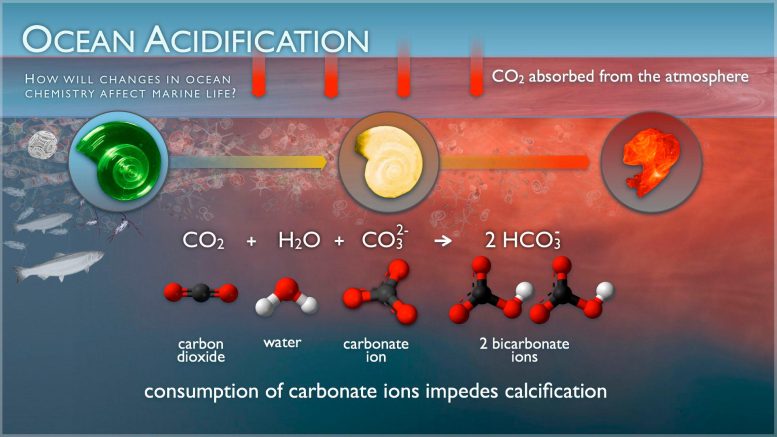
A pteropod shell is shown dissolving over time in seawater with a lower pH. When carbon dioxide is absorbed by the ocean from the atmosphere, the chemistry of the seawater is changed. Credit: NOAA
The Southern Ocean, which surrounds Antarctica, is particularly susceptible to acidification, partly because colder water tends to absorb more CO2. Ocean currents in the area also contribute to the relatively acidic water conditions.
Model Predictions and Marine Protected Areas
Using a computer model, Nissen, Lovenduski, and the team simulated how the seawater of the Southern Ocean would change in the 21st century. They found it would become more acidic by 2100, and the situation would be severe if the world fails to cut emissions.
“It’s not just the top layer of the ocean. The entire water column of the coastal Southern Ocean, even at the bottom, could experience severe acidification,” Nissen said.
The team then investigated the conditions specifically in Antarctica’s marine protected areas (MPAs). Human activities, such as fishing, are restricted in these regions to protect biodiversity. Currently, there are two MPAs in the Southern Ocean, covering about 12% of water in the region. Scientists have proposed designating three more MPAs to an international council in the past years, which would encompass about 60% of the Antarctic Ocean.
The team’s model showed that both adopted and proposed MPAs would experience significant acidification by the end of the century.
For example, under the highest-emission scenario, where the world makes no efforts to cut emissions, the average acidity of the water in the Ross Sea region—the world’s largest MPA off the northern tip of Antarctica—would increase by 104% over 1990s levels by 2100. Under an intermediate emissions scenario, the water would still become 43% more acidic.
“It’s surprising to me how severe ocean acidification would be in these coastal waters,” Nissen said.
Effects on the Food Web
Previous studies have shown that phytoplankton, a group of algae that forms the basis of the marine food web, grow at a slower rate or die out when the water becomes too acidic. Acidic water also weakens the shells of organisms like sea snails and sea urchins. These changes could disrupt the food web, eventually impacting top predators like whales and penguins.
The Weddell Sea is one of the three proposed MPAs located off the coast of the Antarctic Peninsula. Nissen said scientists think the Weddell Sea region could act as a climate change sanctuary for organisms, mainly because this area has the highest levels of sea ice coverage in the Antarctic. The ice shields the ocean from warming and prevents the seawater underneath from absorbing CO2 from the air, thereby reducing the rate of acidification. In addition, the region has little human activity to date.
But the model suggested that as the planet continues to warm, the sea ice will melt, and the Weddell Sea region will experience acidification on par with other MPAs under intermediate to high emission scenarios, but with a slightly delayed progression.
“The result shows that establishing the Weddell Sea region as a protected area should have high priority,” Nissen said.
“As a scientist who typically studies the open ocean, I tend to think of Antarctic coastal areas as a conduit for climate signals to reach the global, deep ocean. This study reminded me that these dynamic Antarctic coastal areas are also themselves capable of rapid change,” Lovenduski said.
The Path Ahead: Emission Reductions
The study suggests that the world could only avoid severe ocean acidification of the Southern Ocean under the lowest emission scenario, where society cuts CO2 emissions quickly and aggressively.
“We still have time to select our emission pathway, but we don’t have much,” Nissen said.
Reference: “Severe 21st-century ocean acidification in Antarctic Marine Protected Areas” by Cara Nissen, Nicole S. Lovenduski, Cassandra M. Brooks, Mario Hoppema, Ralph Timmermann and Judith Hauck, 4 January 2024, Nature Communications.
DOI: 10.1038/s41467-023-44438-x
Additional co-authors of the study included Cassandra Brooks of CU Boulder, and Mario Hoppema, Ralph Timmermann and Judith Hauck at the Alfred Wegener Institute in Bremerhaven, Germany.









“…, the acidity of Antarctica’s coastal waters may double, …”
This is a meaningless statement. Double compared to what? What scale is being used — pH, absolute H+ concentration, or the ratio of hydronium to hydroxyl ions? The oceans are not now acidic. They are alkaline/basic. The quoted statement is like claiming that a woman who is not pregnant may become twice as pregnant. It is grammatical nonsense! Worse yet, it is either purposeful deceit or a demonstration of ignorance of chemistry.
Pure water has very small, equal percentages of hydronium (H+) and hydroxyl (OH-) ions with a ratio of 1:1. They are the basis of the pH scale. There is no imbalance and therefore no tendency for any chemical reactions other than dissolving elements into what has been called the ‘Universal Solvent.’ It is only when the pH is not equal to 7 that oxidation-reduction reactions can take place. However, the potential reactions are different for solutions with a pH7 (basic). Sea water has a nominal pH of around 8.1 and according to the famous geochemist Konrad Krauskopf is unlikely to ever become acidic, only reaching neutrality (pH~7) in stagnant basins rich in hydrogen sulfide. The issue is how much carbonate is dissolved in sea water. If the water is saturated, then there is no tendency to dissolve additional carbonate from, say, the shells of calcifiers. Sea water is highly buffered with carbonate and borate systems that resist changes in pH largely by the spontaneous creation and destruction of carbonate and bicarbonate ions.
“It’s not just the top layer of the ocean. The entire water column of the coastal Southern Ocean, even at the bottom, could experience severe acidification,…”
It seems that the key word here is “coastal.” The degradation of juvenile calcifier shells is typically a problem in coastal areas that experience up-welling of deep, 1,000-year old water that has been enriched in CO2 by bacterial decomposition of the rain of dead plankton coming from the surface waters. The cold and high pressure experienced by the non-anthropogenic CO2 allows it to be retained in solution, decreasing the pH slightly.
From the actual linked article, “Earth system model projections suggest that surface waters in the open Southern Ocean MIGHT become seasonally undersaturated with respect to the more soluble mineral aragonite (Ωarag < 1) by 2035." This is a surprising claim because studies have shown that the seasonal variation in atmospheric CO2 at the South Pole is the lowest on Earth. Besides, global anthropogenic CO2 emissions are relatively constant, being dominated by seasonal bacterial decomposition in the Tundra in the north and by respiration of trees in the Boreal forests of the north. Therefore, it seems unlikely that anthropogenic emissions are driving seasonal variations in the open Southern Ocean. I'd be a lot happier seeing actual probability estimates, along with error bars, instead of weasel words like "could" or "might." After all, their assertions are the result of numerical computer models. It isn't like they don't have the numbers.
Science isn't what it used to be.
http://wattsupwiththat.com/2015/09/15/are-the-oceans-becoming-more-acidic/
“The ice shields the ocean from warming and prevents the seawater underneath from absorbing CO2 from the air, thereby reducing the rate of acidification.”
The ice does nothing to prevent the bacterial production of CO2, and by keeping the CO2 from out-gassing as it would with warming, insures that the carbonic acid stays in the water.
I don’t think that these PhDs have a good grasp of the overall process of the production of CO2. The small anthropogenic production is relatively constant. However, the seasonal ramp-up phase is driven by organic processes while photosynthesis is dormant. When trees leaf out, and phytoplankton bloom, a draw-down phase, of shorter duration than the ramp-up, consumes most, but not all, of the biogenic CO2 in the atmosphere produced the previous Winter/Spring. This small imbalance is what is driving the annual increase of about 2-3PPM CO2.