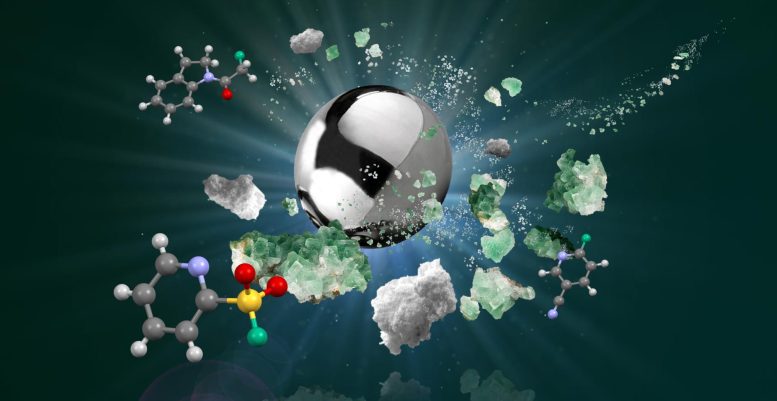
An artistic illustration of the ball-milling process behind the newly developed method for generating fluorochemicals. Credit: Calum Patel
Researchers have developed a safer and more eco-friendly method for producing fluorochemicals, eliminating the use of hazardous hydrogen fluoride gas. The new technique, inspired by biomineralization processes, could revolutionize the fluorochemical industry and has led to the founding of a company, FluoRok, to commercialize the technology.
- For the first time, Oxford chemists have generated fluorochemicals – critical for many industries – without the use of hazardous hydrogen fluoride gas.
- The innovative method was inspired by the biomineralization process that forms our teeth and bones.
- The results were published in the leading journal Science.
A team of chemists has developed an entirely new method for generating critically important fluorochemicals that bypasses the hazardous product hydrogen fluoride (HF) gas. The findings, published on July 20 in Science, could achieve an immense impact in improving the safety and carbon footprint of a growing global industry.
Fluorochemicals are a group of chemicals that have a wide range of important applications – including polymers, agrochemicals, pharmaceuticals, and the lithium-ion batteries in smartphones and electric cars – with a $21.4 billion global market in 2018. Currently, all fluorochemicals are generated from the toxic and corrosive gas hydrogen fluoride (HF) in a highly energy-intensive process. Despite stringent safety regulations, HF spills have occurred numerous times in the last decades, sometimes with fatal accidents and detrimental environmental effects.
To develop a safer approach, a team of chemists at the University of Oxford alongside colleagues in Oxford spin-out FluoRok, University College London, and Colorado State University, took inspiration from the natural biomineralization process that forms teeth and bones. Normally, HF itself is produced by reacting a crystalline mineral called fluorspar (CaF2) with sulfuric acid under harsh conditions, before it is used to make fluorochemicals. In the new method, fluorochemicals are made directly from CaF2, completely bypassing the production of HF: an achievement that chemists have sought for decades.
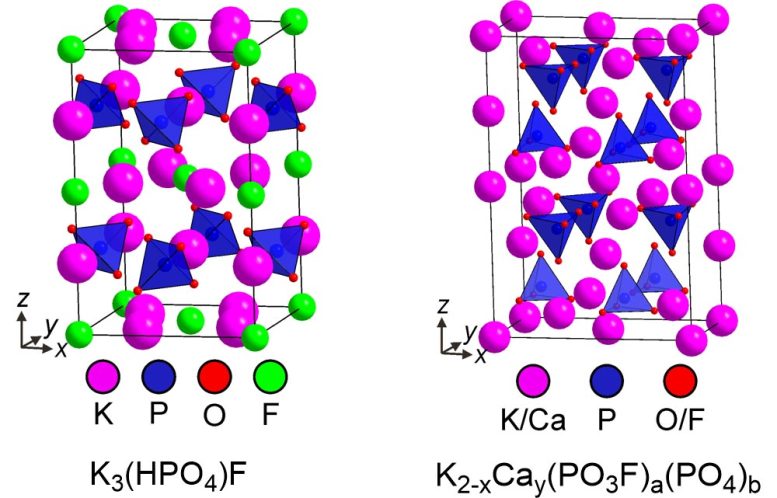
Using high precision techniques, such as X-ray diffraction, the researchers unlocked key insights into the composition of Fluoromix and structures of the fluorinating species. The diagram shows structures of crystalline constituents of Fluoromix, which serve as fluorinating reagents. Credit: Prof. Michael Hayward
In the novel method, solid-state CaF2 is activated by a biomineralization‑inspired process, which mimics the way that calcium phosphate minerals form biologically in teeth and bones. The team ground CaF2 with powdered potassium phosphate salt in a ball-mill machine for several hours, using a mechanochemical process that has evolved from the traditional way that we grind spices with a pestle and mortar.
The resulting powdered product, called Fluoromix, enabled the synthesis of over 50 different fluorochemicals directly from CaF2, with up to 98% yield. The method developed has the potential to streamline the current supply chain and decrease energy requirements, helping to meet future sustainability targets and lower the carbon footprint of the industry.
Excitingly, the solid-state process developed was just as effective with acid grade fluorspar (> 97%, CaF2) as it was with synthetic reagent grade CaF2. The process represents a paradigm shift for the manufacturing of fluorochemicals across the globe and has led to the creation of FluoRok, a spin‑out company focusing on the commercialization of this technology and the development of safe, sustainable, and cost-effective fluorinations. The researchers hope that this study will encourage scientists around the world to provide disruptive solutions to challenging chemical problems, with the prospect of societal benefit.
Calum Patel, from the Department of Chemistry, University of Oxford, and one of the lead authors of the study, says:
“Mechanochemical activation of CaF2 with a phosphate salt was an exciting invention because this seemingly simple process represents a highly effective solution to a complex problem; however, big questions on how this reaction worked remained. Collaboration was key to answering these questions and advancing our understanding of this new, unexplored area of fluorine chemistry. Successful solutions to big challenges come from multidisciplinary approaches and expertise, I think the work really captures the importance of that.”
Lead author Professor Véronique Gouverneur FRS, from the Department of Chemistry, University of Oxford, who conceived and led this study says:
“The direct use of CaF2 for fluorination is a holy grail in the field, and a solution to this problem has been sought for decades. The transition to sustainable methods for the manufacturing of chemicals, with reduced or no detrimental impact on the environment, is today a high-priority goal that can be accelerated with ambitious programs and a total re-think of current manufacturing processes. This study represents an important step in this direction because the method developed in Oxford has the potential to be implemented anywhere in academia and industry, minimize carbon emissions e.g. by shortening supply chains, and offer increased reliability in light of the fragility of global supply chains.”
Reference: “Fluorochemicals from fluorspar via a phosphate-enabled mechanochemical process that bypasses HF” by Calum Patel, Emy André-Joyaux, Jamie A. Leitch, Xabier Martínez de Irujo-Labalde, Francesco Ibba, Job Struijs, Mathias A. Ellwanger, Robert Paton, Duncan L. Browne, Gabriele Pupo, Simon Aldridge, Michael A. Hayward and Véronique Gouverneur, 20 July 2023, Science.
DOI: 10.1126/science.adi1557
About the team
Calum Patel completed his Master’s degree in Chemistry at Imperial College London, and has worked at the University of British Columbia and at F. Hoffmann La Roche in Basel on late-stage fluorination. He has a keen interest in the development of novel fluorination methods as part of the Gouverneur research group at the University of Oxford.
Prof Véronique Gouverneur FRS is the Waynflete Professor of Chemistry at the University of Oxford. She obtained a PhD in chemistry at the Université Catholique de Louvain (LLN, Belgium) and completed a postdoctoral position at the Scripps Research Institute (California, USA). She then held a position at the University Louis Pasteur in Strasbourg (France) and started her independent research career at the University of Oxford in 1998. She has received numerous prizes and distinctions for her research (Arthur C. Cope Award 2022, Moissan Prize 2021, International Honorary Member of the American Academy of Arts & Sciences) and has over 220 peer-reviewed publications and 15 patents.
Prof Michael Hayward is a Professor of Inorganic Chemistry and Tutorial Fellow at Somerville College. He completed his D.Phil. in Oxford in 1999 and after completing a period of post‑doctoral research at Princeton University he returned to Oxford in 2002, initially as a Royal Society University Research Fellow and JRF at Merton College, before being appointed to a fellowship Somerville College in 2004. His research focuses on the synthesis and characterization of novel solid-state compounds.






I’m glad that producing fluorochemicals can be safer. Should we even be producing them??? What are the negative effects–especially genetically–of these products.