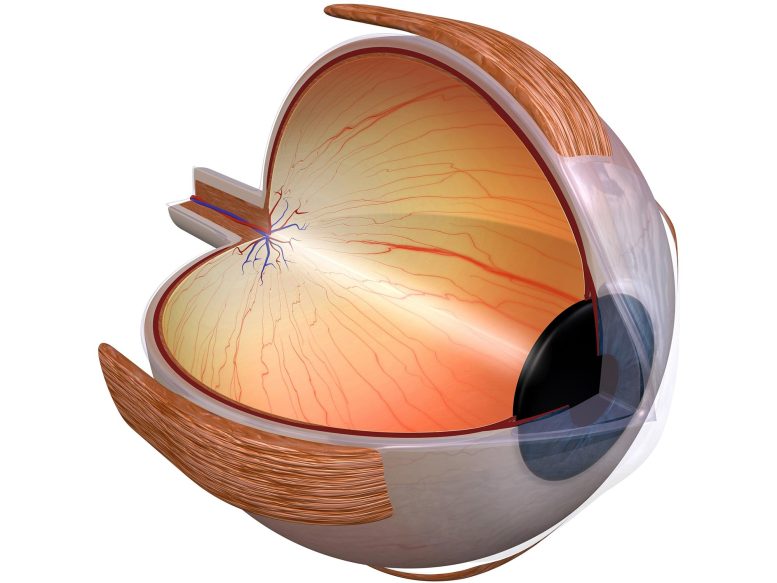
Researchers mapped the organization of human retinal cell chromatin, resulting in a comprehensive gene regulatory network that provides insights into the regulation of gene expression in both rare and common eye diseases.
NIH researchers reveal new insights on how genetic architecture determines gene expression, tissue-specific function, and disease phenotype in blinding diseases.
National Eye Institute (NEI) scientists have mapped the organization of human retinal cell chromatin. These are the fibers that package 3 billion nucleotide-long DNA molecules into compact structures that fit into chromosomes within each cell’s nucleus. The resulting comprehensive gene regulatory network provides insights into the regulation of gene expression in general, and in retinal function, in both rare and common eye diseases. The study will be published today (October 7, 2022) in the journal Nature Communications.
“This is the first detailed integration of retinal regulatory genome topology with genetic variants associated with age-related macular degeneration (AMD) and glaucoma, two leading causes of vision loss and blindness,” said Anand Swaroop, Ph.D., the study’s lead investigator. He is senior investigator and chief of the Neurobiology Neurodegeneration and Repair Laboratory at the NEI, part of the National Institutes of Health (NIH).
Adult human retinal cells are highly specialized sensory neurons that do not divide and are therefore relatively stable. This makes them useful for exploring how the chromatin’s three-dimensional structure contributes to the expression of genetic information.
Chromatin fibers package long strands of DNA, which are spooled around histone proteins and then repeatedly looped to form highly compact structures. All those loops create multiple contact points where genetic sequences that code for proteins interact with gene regulatory sequences, such as super-enhancers, promoters, and transcription factors.
Chromatin is a mixture of DNA and proteins that form the chromosomes found in the cells of humans and other higher organisms. Many of the proteins — namely, histones — package the massive amount of DNA in a genome into a highly compact form that can fit in the cell nucleus.
For a long time, such non-coding sequences were considered “junk DNA.” However, more advanced studies have demonstrated ways that these sequences control which genes get transcribed and when. This has shed light on the specific mechanisms by which non-coding regulatory elements exert control even when their location on a DNA strand is remote from the genes they regulate.
Using deep Hi-C sequencing, a tool used for studying 3D genome organization, the scientists created a high-resolution map that included 704 million contact points within retinal cell chromatin. Maps were constructed using post-mortem retinal samples from four human donors.
The research team then integrated that chromatin topology map with datasets on retinal genes and regulatory elements. What emerged was a dynamic picture of interactions within chromatin over time, including gene activity hot spots and areas with varying degrees of insulation from other regions of DNA.
They found distinct patterns of interaction at retinal genes suggesting how chromatin’s 3D organization plays an important role in tissue-specific gene regulation.
“Having such a high-resolution picture of genomic architecture will continue to provide insights into the genetic control of tissue-specific functions,” Swaroop said.
Moreover, similarities between mice and human chromatin organization suggest conservation across species, underscoring the relevance of chromatin organizational patterns for retinal gene regulation. More than a third (35.7%) of gene pairs interacting through a chromatin loop in mice also did so in human retina.
The scientists integrated the chromatin topology map with data on genetic variants identified from genome-wide association studies for their involvement in age-related macular degeneration (AMD) and glaucoma, two of the leading causes of vision loss and blindness. The findings point to specific candidate causal genes involved in those diseases.
The integrated genome regulatory map will also assist in evaluating genes associated with other common retina-associated diseases such as diabetic retinopathy, determining missing heritability, and understanding genotype-phenotype correlations in inherited retinal and macular diseases.
Reference: “High-resolution genome topology of human retina uncovers super enhancer-promoter interactions at tissue-specific and multifactorial disease loci” by Claire Marchal, Nivedita Singh, Zachary Batz, Jayshree Advani, Catherine Jaeger, Ximena Corso-Díaz and Anand Swaroop, 7 October 2022, Nature Communications.
DOI: 10.1038/s41467-022-33427-1
The study was supported by the NEI Intramural Research Program, grants ZIAEY000450 and ZIAEY000546.








Be the first to comment on "New Insights Into Eye Diseases: 3D Map Reveals DNA Organization Within Human Retina Cells"