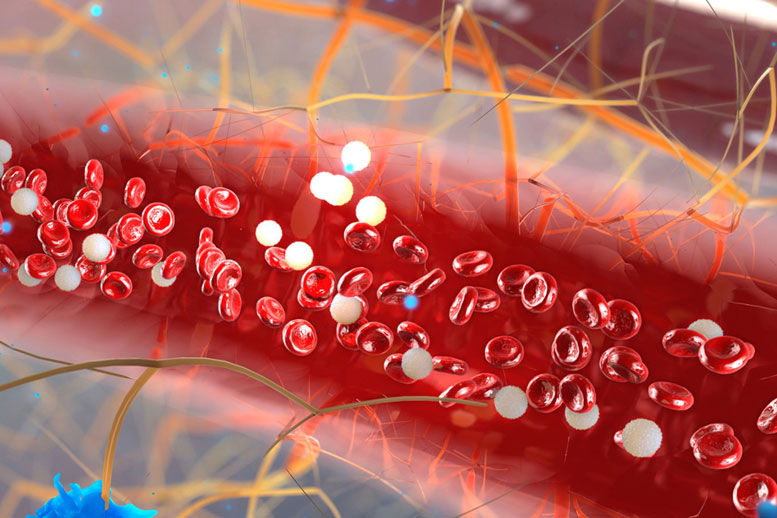
MIT scientists created a microfluidic device to extract plasma cells from blood, offering multiple myeloma patients a less painful test.
A team of scientists at MIT has developed a microfluidic device that isolates plasma cells from blood rather than from bone marrow. The new technique may provide patients with a less painful test for multiple myeloma.
Multiple myeloma is a cancer of the plasma cells, which are white blood cells produced in the bone marrow that churn out antibodies to help fight infection. When plasma cells become cancerous, they produce abnormal proteins, and the cells can build up in bone marrow, ultimately seeping into the bloodstream.
Multiple myeloma is typically diagnosed through a bone marrow biopsy, in which a needle is inserted near a patient’s hip bone to suck out a sample of bone marrow — a painful process for many patients. Clinicians can then isolate and analyze the plasma cells in the bone marrow sample to determine if they are cancerous.
Currently, there is no way to easily identify plasma cells that have escaped into the bloodstream. Circulating plasma cells are not normally found in healthy people, and the ability to identify these cells in the blood could enable doctors to diagnose and track the progression of multiple myeloma.
Now engineers at MIT have devised a microfluidic technique to capture and count circulating plasma cells from small samples of blood. The technique relies on conventional blood draws and may provide patients with a less painful test for multiple myeloma.
“Procedures of the traditional tissue biopsy are painful, associated with complications such as potential infections, and often available only in central hospitals which require patients to travel long distances,” says former MIT postdoc Mohammad Qasaimeh. “Capturing plasma cells from blood samples can serve as a liquid biopsy, which can be performed in clinics as often as required, and serve as a diagnostic and prognostic test during and after chemotherapy treatment. Moreover, captured cells can be used for drug testing and thus serve as a tool for personalized medicine.”
Qasaimeh and his colleagues have published their results in the journal Scientific Reports. His co-authors include former students Yichao Wu and Suman Bose, Rao Prabhala, an instructor in medicine at Dana-Farber Cancer Institute and Harvard Medical School; Jeffrey Karp, an associate professor in the Harvard-MIT Division of Health Sciences and Technology; and Rohit Karnik, an associate professor in MIT’s Department of Mechanical Engineering.
A herringbone trap
The new technique builds on a microfluidic design that was previously developed by George Whitesides, a professor of chemistry at Harvard University. Whitesides and his colleagues fabricated a small microchip, the channel of which they etched with repeating, V-shaped grooves, similar to a herringbone pattern. The grooves cause any fluid flowing through the microchip to swirl about in eddies, rather than passing straight through. The cells within the fluid, therefore, have a higher chance of making contact with the floor of the device, as first shown by Memhmet Toner at Massachusetts General Hospital.
Researchers including Karnik have since reproduced this microfluidic design, coating the microchip’s floor with certain molecules to attract cells of interest.
Karnik’s team used the microfluidic herringbone design to capture circulating plasma cells in their latest work. They coated the channels of a microchip, about the size of a glass slide, with CD138, an antibody that is also expressed on the membranes of plasma cells. The team then flowed small, 1-milliliter samples of blood through the device. The herringbone grooves circulated the blood in the microfluidic channels, where the antibodies, acting as tiny Velcro pads, grabbed onto any passing plasma cells while letting the rest of the blood flow out of the device.
Once the cells were isolated in the microchip, the scientists could count the cells and determine the kinds of antibodies that each cell secretes.
“With the ease of a blood draw”
Using blood samples from healthy donors as well as patients with the disease, the researchers tested the device. After counting the number of cells captured in each sample, they observed very low numbers of circulating plasma cells in healthy samples — about two to five cells per milliliter of blood — versus substantially higher counts in patients diagnosed with multiple myeloma, of about 45 to 184 cells per milliliter.
The team also analyzed the captured plasma cells to determine the type of antibodies they produced. Plasma cells can generate one of two kinds of antibodies, known as kappa- and lambda-type. In addition to conducting bone marrow biopsies, clinicians can analyze blood samples for the ratio of these two antibodies, which can be an indicator of how the disease is progressing.
Karnik and his colleagues determined the ratio of plasma cells producing kappa- and lambda-type antibodies, and compared them to conventional blood tests for the same antibodies, for both healthy subjects and patients with multiple myeloma. They found both sets of results matched, validating the microfluidic device’s accuracy.
Surprisingly, the team noted that patients who were in remission exhibited higher counts of circulating plasma cells than healthy donors. These same patients had shown normal ratios of antibodies in conventional blood tests. Karnik says that the group’s new device may reveal more subtle information about a patient’s state, even in remission.
“When patients go into remission, their antibody levels can look normal,” Karnik says. “But we detect a level of circulating plasma cells that is above the baseline. It’s hard to tell whether these cells are cancerous, but at least this technique is giving us more information. With the ease of a blood draw, this may enable us to track cancer in a much better way.”
Karnik adds that in the future, scientists may use the group’s design to perform genetic tests on the captured cells, or to look for mutations in the cells that may further characterize the disease.
“We can capture and stain these cells in the device, which opens the possibility of studying whether there are new mutations in the cells,” Karnik says. “With cancers like multiple myeloma, even for patients in remission, cancer can recur. Detecting the level or mutation of plasma cells in blood might provide an early detection method for these patients.”
This research was supported, in part, by the National Institutes of Health and the Al Jalila Foundation.
Reference: “Isolation of Circulating Plasma Cells in Multiple Myeloma Using CD138 Antibody-Based Capture in a Microfluidic Device” by Mohammad A. Qasaimeh, Yichao C. Wu, Suman Bose, Anoop Menachery, Srikanth Talluri, Gabriel Gonzalez, Mariateresa Fulciniti, Jeffrey M. Karp, Rao H. Prabhala and Rohit Karnik, 4 April 2017, Scientific Reports.
DOI: 10.1038/srep45681

Be the first to comment on "New Microfluidic Device Isolates Plasma Cells from Blood Instead of Bone Marrow"