Overactive populations of osteoclasts may result in a variety of disorders such as osteoporosis, arthritis, and cancer.
A new function for a protein that regulates osteoclasts—the cells that break down bone—has been discovered by researchers, and it may pave the way for the creation of new treatments to prevent bone loss.
Bone remodeling in the body is a delicate balancing act between osteoblasts, cells who produce bone, and osteoclasts, cells who break it down. Diseases like osteoporosis, arthritis, and periodontitis all cause bone loss and are associated with an increase in osteoclast activity.
Researchers from the University of Pennsylvania and colleagues offer new insight on the regulation of osteoclasts in a recent study that was published in the journal Proceedings of the National Academy of Sciences, potentially shedding light on the imbalances that may lead to disease. The study identified the protein IFT80 as a crucial component in controlling osteoclast populations. The researchers also discovered that mice missing IFT80 had larger-than-expected populations of osteoclasts and developed severe osteopenia.
“When you think about translation to the clinic, we believe this finding is very important,” says Shuying (Sheri) Yang, an associate professor in Penn’s School of Dental Medicine and the study’s senior author. “As we begin to understand the mechanism and the gene function of IFT80, we may be able to consider it as a potential therapeutic target. For example, a DNA- or mRNA-based therapy that introduced this protein could help treat certain bone diseases.”
After seeing an earlier study in Nature Cell Biology, Yang and colleagues became interested in IFT80. That study discovered that IFT, or intraflagellar proteins, are involved in T cell protein transport. Yang’s lab studies these proteins and the finding caught her attention since T cells and osteoclasts are both produced from hematopoietic stem cells, the precursors of blood cells.
IFTs aid in the formation of cilia, which are antenna-like sensory organs that extend from cells, by transporting proteins from the cilia’s base to its tip and back again. Yang and colleagues previously demonstrated that IFTs serve key roles in regulating osteoblasts and chondrocytes, cells that maintain cartilage, from mesenchymal stem cells, which produce and maintain bone, cartilage, and other tissue types.
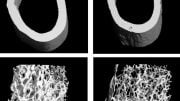
To explore the role of IFT80 specifically in osteoclasts, Yang’s group developed a knockout mouse line that lacked the protein in precursors of osteoclasts. Notably, they found these animals had significantly lower bone volume compared to normal mice, and their osteoclasts nearly doubled in number. “It was a dramatic change,” Yang says.
The researchers found that IFT80 prevents osteoclast precursors from giving rise to the bone resorbing cells and inhibits osteoclast maturation.
Further experimentation indicated that IFT80 interacted with a protein called Cbl-b in the protein degradation pathway regulated by the small regulatory protein ubiquitin in osteoclasts. Yang’s team found that IFT80 prevents the breakdown of Cbl-b, and Cbl-b normally degrades another protein called TRAF6. TRAF6 normally promotes osteoclast production, so by degrading TRAF6, IFT80 inhibits osteoclast differentiation.
Downstream of TRAF6, the research team also found evidence that IFT80 suppresses a signaling pathway governed by the proteins RANKL and RANK.
To test the idea of IFT80 being a potential target for intervention in bone loss disorders, the researchers overexpressed IFT80 in a mouse model that normally experiences overactive osteoclasts-caused bone loss. Doing so effectively tamped down RANK/RANKL activation, lowered osteoclast production, and increased bone volume in the mice.
The study is the first to link IFT80 with a role in osteoclasts and to find that IFT80 controls a protein degradation pathway and serves as a negative regulator during osteoclast differentiation. These features makes it a valuable target for potential therapeutic intervention, says Yang.
“Right now there is a lot of interest in how the body promotes osteoclast differentiation,” she says. “With so many diseases related to excess bone loss—osteoporosis, periodontitis, rheumatoid arthritis, even fractures—there is a big need to find ways to address bone loss and restore balance in bone remodeling.”
References:
“IFT80 negatively regulates osteoclast differentiation via association with Cbl-b to disrupt TRAF6 stabilization and activation” by Vishwa Deepak, Shu-ting Yang, Ziqing Li, Xinhua Li, Andrew Ng, Ding Xu, Yi-Ping Li, Merry Jo Oursler and Shuying Yang, 21 June 2022, Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.2201490119
“Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse” by Francesca Finetti, Silvia Rossi Paccani, Maria Giovanna Riparbelli, Emiliana Giacomello, Giuseppe Perinetti, Gregory J. Pazour, Joel L. Rosenbaum, and Cosima T. Baldari, 25 October 2009, Nature Cell Biology.
DOI: 10.1038/ncb1977
The study was funded by the National Institutes of Health.


Be the first to comment on "New Treatment Target Could Counter Bone Loss"