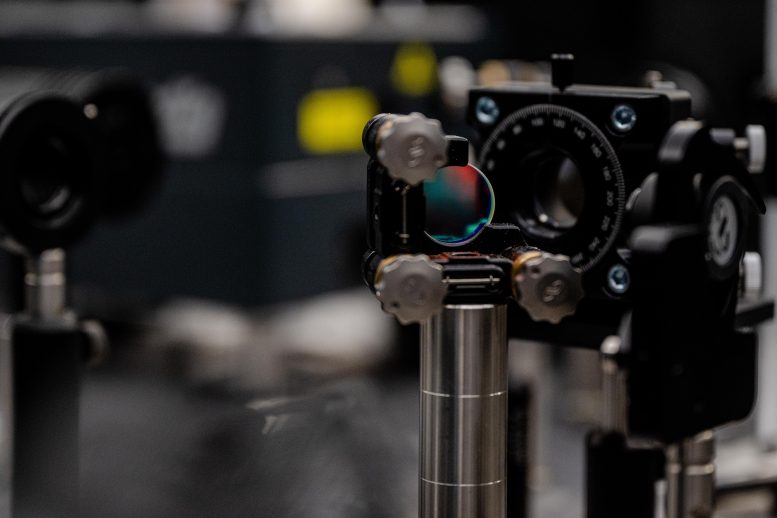
The study was only made possible by the new laser laboratories in the ZEMOS research building, where all external interference signals are minimized. Credit: RUB, Marquard
In specific molecules known as photoacids, light excitation can trigger a local release of a proton. This results in an abrupt shift in the solution’s pH level, acting as a rapid switch crucial to numerous chemical and biological processes. The precise events occurring during proton release remained uncertain until recently. Researchers from the Cluster of Excellence Ruhr Explores Solvation (RESOLV) at Ruhr University Bochum, Germany, have now successfully observed this process using cutting-edge technology in an experiment.
The researchers observed a beating between the solute and solvent that set off a minuscule tremor lasting just three to five picoseconds prior to the proton’s detachment. Their findings are detailed in the journal Chemical Science.
So far, the focus has been on dye or base
One of the most studied so-called photoacids is pyranine, the fluorescent dye used, for instance, in yellow highlighters. “Despite a wealth of experimental studies, the process that is at the very beginning of proton detachment still remained the subject of controversial debate,” reports Professor Martina Havenith, spokesperson for RESOLV. However, the entire detachment process also happens on a time scale of only 90 picoseconds. A picosecond corresponds to a millionth of a millionth of a second.

Claudius Hoberg and Martina Havenith (right) conducted the research in the ZEMOS research building. Credit: RUB, Marquard
While previous studies focused mainly on the change of the dye after light excitation, the team was able to observe the change of the solvent, in this case, water, during this process for the first time. This was achieved with the help of a newly developed technique, “Optical Pump THz Probe Spectroscopy”.
Ultra-fast energy transmission
“We were able to follow how there is an oscillation at the beginning, which then subsides subsequently,” describes Martina Havenith. “It is exciting to see that the solvent response that promotes excited-state proton transfer could be caught in the act.” The initial ultrafast transfer of energy within less than a picosecond causes a restructuring of the water molecules in the immediate vicinity and opens the way for the subsequent proton migration.
The detection was made possible by the new laser laboratories in the ZEMOS research building. There, all external interference signals, such as those caused by electromagnetic radiation, temperature, and humidity fluctuations, are minimized. Only then is it possible to detect even the tiniest tremors in a water jet with the dye.
Reference: “Caught in the act: real-time observation of the solvent response that promotes excited-state proton transfer in pyranine” by Claudius Hoberg, Justin J. Talbot, James Shee, Thorsten Ockelmann, Debasish Das Mahanta, Fabio Novelli, Martin Head-Gordon and Martina Havenith, 16 March 2023, Chemical Science.
DOI: 10.1039/D2SC07126F
The accompanying simulations were carried out at the University of California at Berkeley in the research group of Martin Head-Gordon. It belongs to CALSOLV, the sister institute of RESOLV.
The study was funded by the German Research Foundation, the European Research Council, and Mercator Research Center Ruhr.







Be the first to comment on "Picosecond Phenomenon: Observing Proton Release in Photoacids"