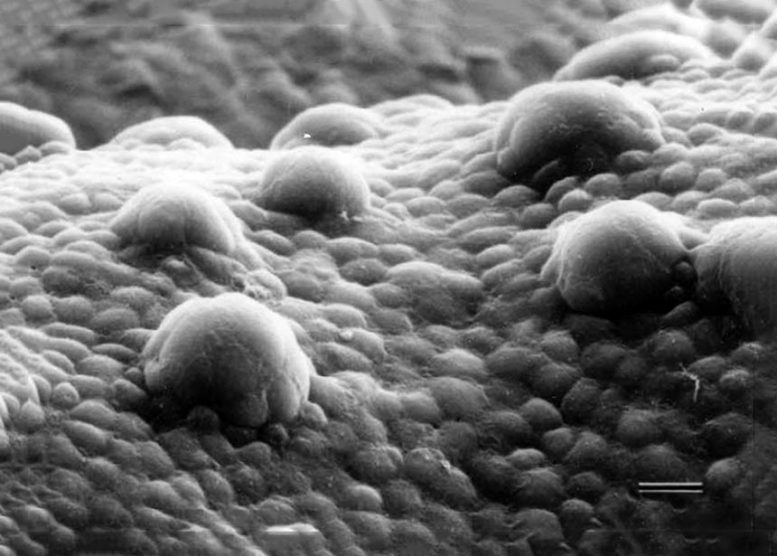
Fungiform taste papillae, small structures or “bumps” found on the upper surface of the front two thirds of the tongue. Credit: UGA
COVID-19 does not directly damage taste bud cells. First study to show taste bud cell immunity from contact with virus particle.
A new study from the Regenerative Bioscience Center at the University of Georgia is the first to suggest that COVID-19 does not directly damage taste bud cells.
Contrary to previous studies that have shown damage may be caused directly by the virus particle, the researchers, led by Hongxiang Liu, associate professor of animal and dairy science in UGA’s College of Agricultural and Environmental Sciences, found that taste loss is likely caused indirectly by events induced during COVID-19 inflammation.
An increasing number of COVID-19 patients have reported losses of smell and/or taste, prompting the CDC to add it to the growing list of symptoms for COVID-19. Recent research shows 20%-25% of patients now report a loss of taste.
“More alarming is the rate of patients reporting loss of taste at a later date, sometime after exposure to the virus,” said Liu. “This is something we need to keep a careful eye on.”
Published in ACS Pharmacology & Translational Science, the study further indicates that taste bud cells are not vulnerable to SARS-CoV-2 infection, because most of them do not express ACE2, a gateway that the virus uses to enter the body.
“This study isn’t the first to study ACE2 expression in the oral cavity,” said Liu. “But it is the first to show, specifically in relation to coronavirus and taste bud cell survival, that there are likely other cell death mechanisms at play.”
Liu and her colleagues wanted to find out whether ACE2 was expressed specifically in taste bud cells, as well as when this receptor first emerges on oral tissue cells during fetal development, by studying mice as a model organism.
Although the mouse version of ACE2 isn’t susceptible to SARS-CoV-2, studying where it’s expressed in mice could still help clarify what’s happening when people become infected and lose the sense of taste, given that mouse and human share similar expression patterns of genes.
“Mice have a different cellular copy of ACE2, making them impervious to SARS-CoV-2 infection,” said Liu. “A logical first step was to genetically engineer a model to examine the ACE2 receptor expression in wild type mice, to provide insights into what happens in people.”
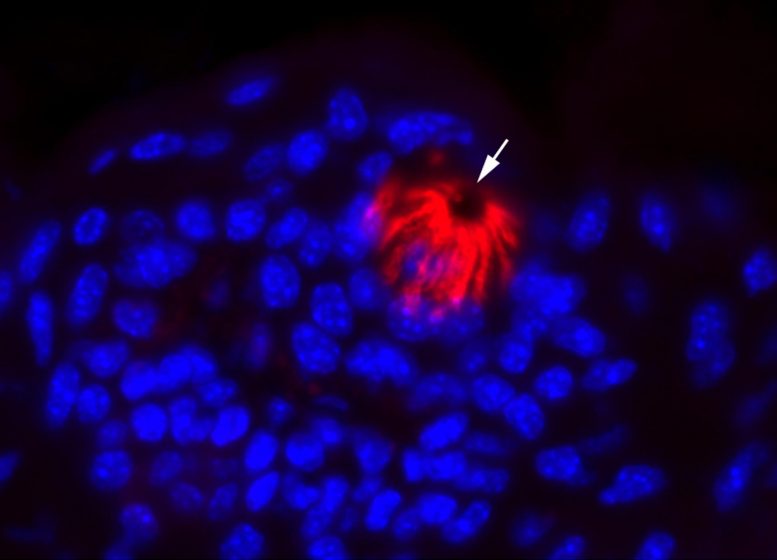
By analyzing data from oral cells of adult mice, the researchers found that ACE2 was enriched in cells that give the tongue its rough surface, but couldn’t be found in most taste bud cells. That means the virus probably does not affect taste loss through direct infection of these cells.
“It’s clear from the data, that future designs of therapeutics directed at ACE2 receptors would likely not be as effective in treating taste loss of patients suffering from COVID-19,” said Liu.
According to the team, more researchers have jumped into studying coronavirus and have published more data for smell loss than taste.
“Anosmia coronavirus research is being published at a faster pace,” said Liu. “This is the only COVID-19 research that we know of, that involves the mechanisms of taste loss. Taste loss in the tongue is more complex and harder to validate, because of the complexity of cells, tissue structures, and the limited expression level of the ACE2 receptor.
Reference: “SARS-CoV-2 Receptor ACE2 Is Enriched in a Subpopulation of Mouse Tongue Epithelial Cells in Nongustatory Papillae but Not in Taste Buds or Embryonic Oral Epithelium” by Zhonghou Wang, Jingqi Zhou, Brett Marshall, Romdhane Rekaya, Kaixiong Ye and Hong-Xiang Liu, 23 July 2020, ACS Pharmacology & Translational Science.
DOI: 10.1021/acsptsci.0c00062







Be the first to comment on "Researchers Examined What COVID-19 Does to Taste Bud Cells – Here’s What They Found"