
Alzheimer’s disease is a progressive neurodegenerative disorder that affects memory, thinking, and behavior. It is the most common cause of dementia in older adults and results in a decline in cognitive function and independence.
Researchers from Drexel University have shed new light on the role of the Tip60 enzyme in genetic disruptions that contribute to Alzheimer’s disease.
A team of scientists from Drexel University has discovered a new brain regulation mechanism that plays a crucial role in producing the proper proteins that maintain optimal brain health. The malfunctioning of this mechanism may be an early contributor to Alzheimer’s disease.
The brain’s ability to constantly adapt to new stimuli and form new memories is due to the versatility of brain cells. These cells have the ability to generate various functional versions of the same protein through a process known as alternative RNA splicing.
Recent studies have reported defects in RNA splicing of genes in the brains of Alzheimer’s patients, which has led to the conclusion that splicing disruptions are considered an indicator of Alzheimer’s disease. The causes for these splicing disruptions in the brain are still unknown, which has hindered the development of treatments focused on this defect.
The team led by Akanksha Bhatnagar, a Ph.D. student, and Felice Elefant, Ph.D., a professor, who are biology researchers in Drexel’s College of Arts and Sciences, is the first to uncover the role of the Tip60 enzyme in binding to certain RNA in the brain to control how they are spliced.
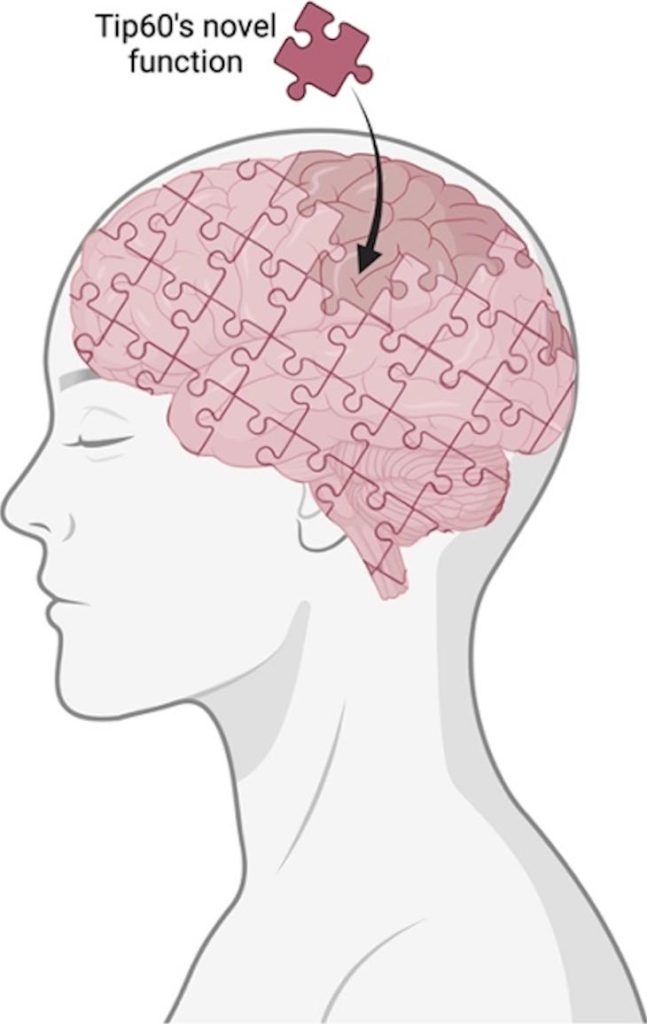
Tip60’s novel function is a critical piece in the brain puzzle that brings us one step closer to understanding the big picture behind Alzheimer’s Disease. Credit: Image was generated using BioRender.
This function is particularly important, the researchers suggest, because such RNA splicing ultimately generates the protein diversity required for learning and memory and the majority of RNA that Tip60 binds to are encoded by genes implicated in Alzheimer’s disease progression.
A 2018 Drexel study by Elefant’s lab showed that restoration of the enzyme Tip60 created a balancing of the enzymes in the brain and reversed symptoms in an Alzheimer’s model system. Building on that study, Elefant, Bhatnagar, and their team — whose findings were published in the Journal of Neuroscience — discovered that Tip60 doesn’t just regulate gene activation to make RNA, it also regulates the way RNA is spliced to generate diverse protein variants, which has been shown to contribute to the reversal of Alzheimer’s symptoms.
“We have previously shown that Tip60 enzyme levels are depleted in Alzheimer’s disease brains and this depletion results in some genes getting inactivated. However, with this new RNA splicing function, we now show Tip60 is also not present to bind to the RNA to allow for appropriate splicing in the brain and may be causing some of the splicing defects observed in Alzheimer’s disease,” said Elefant.
The researchers found that restoring depleted amounts of Tip60 in Alzheimer’s models, not only rescues gene activation, but also partially protects against splicing disruptions — demonstrating that the Tip60 enzyme can be a drug target to protect against two different processes that go awry in Alzheimer’s.
“RNA is essential in coding, decoding, regulation, and the expression of genes,” said Akanksha Bhatnagar. “In the Alzheimer’s brain, not only is the production of RNA being turned off, but also the way the RNA is being assembled to generate proteins is not correct. This is a critical piece in the Alzheimer’s disease puzzle that has opened new doors for understanding disease causes.”
The Elefant Lab at Drexel also explores how environmental changes can impact Alzheimer’s disease.
“More than 95% of Alzheimer’s cases do not have a clear genetic link with parents, they are arising sporadically due to external factors, or epigenetics,” said Bhatnagar. “We want to know how and why environmental changes can impact Alzheimer’s and one of the ways this can happen is through the enzyme we study, Tip60.”
These findings could lead to important developments for drug design and the treatment of Alzheimer’s, according to the team.
“If we can figure out what genes are being altered before deficits occur, there is a possibility that these RNA variations could serve as biomarkers to identify Alzheimer’s disease early on,” said Bhatnagar.
Reference: “Tip60’s Novel RNA-Binding Function Modulates Alternative Splicing of Pre-mRNA Targets Implicated in Alzheimer’s Disease” by Akanksha Bhatnagar, Keegan Krick, Bhanu Chandra Karisetty, Ellen M. Armour, Elizabeth A. Heller and Felice Elefant, 29 March 2023, JNeurosci.
DOI: 10.1523/JNEUROSCI.2331-22.2023
The study was funded by the National Institutes of Neurological Disorders and Stroke of the NIH.





Be the first to comment on "Researchers Solve One Piece of Alzheimer’s Puzzle"