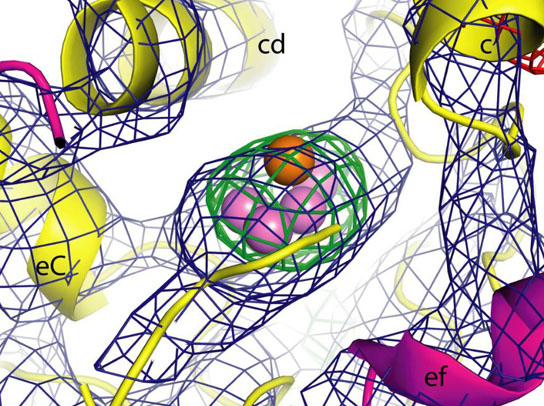
This illustration shows an electron-density image of a Photosystem II molecular cluster, based on data gathered from an LCLS experiment. Protein is displayed as yellow, the violet spheres are manganese, and the orange orb is calcium. Credit: Image adapted from the Proceedings of the National Academy of Sciences
Scientists from the Berkeley Lab and SLAC National Accelerator Laboratory are using the Linac Coherent Light Source to research a tiny cluster of molecules that is integral to an important stage of photosynthesis known as Photosystem II. With a better understanding of this process, researchers may one day develop technologies that can replicate nature and produce cheaper and more efficient fuels.
The molecular power plants that carry out photosynthesis are at the root of a scientific quest to learn how they channel energy from sunlight to split water into oxygen and hydrogen.
Understanding these fundamental processes could help scientists develop technologies that replicate nature’s handiwork to produce cheaper and more efficient fuels.
Now, an international research team led by scientists at Lawrence Berkeley National Laboratory and SLAC National Accelerator Laboratory has used a powerful X-ray laser to shine new light on a tiny cluster of molecules that is integral to an important stage of photosynthesis known as Photosystem II. The results were published June 4 in the Proceedings of the National Academy of Sciences.
The team crystallized the molecular clusters, suspended the crystals in liquid and injected them into the path of SLAC’s X-ray laser, the Linac Coherent Light Source (LCLS). Laser light diffracting off the crystals formed patterns that were used to reconstruct the composition and atomic structure of the clusters.
In Photosystem II, plants absorb photons from sunlight to drive chemical reactions that oxidize water, splitting water molecules into hydrogen and oxygen. Electrons extracted from the water power the photosynthetic process, generating almost all the food and energy that life on Earth depends on.
Junko Yano, staff scientist in the Physical Biosciences Division at Berkeley Lab and one of three leaders of the research team, said it is the very last step in Photosystem II – the point at which oxygen is released – that has proved most difficult for researchers to observe.
The successful imaging of the crystallized molecules at LCLS gives the team confidence that it can use the instrument to study other steps of the photosynthetic process, she said. What’s more, the imaging was done at room temperature and without the need to freeze samples, which can distort the results.
Researchers achieved a resolution of 6.5 Ångstroms – an Ångstrom is one ten-billionth of a meter – for the structure of the crystallized clusters that they used in their data. Some previous experiments have achieved higher resolutions, but with frozen crystals that may have been altered by X-rays.
Jan Kern, a research scientist at SLAC and Berkeley Lab who was the lead author on the paper, said, “We hope that with improved samples, in the future we will be able to get to a higher resolution.”
Yano said one of the goals of photosynthesis research is developing a clean and affordable fuel out of common molecules like water and carbon dioxide. “Is there any way to directly make a liquid fuel using sunlight and water as a source? How are we going to do it? How efficiently can we do it?” she said. “That’s what we’re going to learn from nature.”
LCLS is proving a valuable research tool in biological research because its ultrashort, ultrabright pulses can provide data in the quadrillionths of a second before the sample is destroyed by the powerful X-ray radiation.
“Having a probe where you can study the Photosystem II mechanism in real time, with a technique where you can probe before the sample is destroyed, might really be the key to solving this question,” said team co-leader Uwe Bergmann, deputy director for the LCLS and a senior staff scientist at SLAC.
He said the research highlighted in the paper represents an expansion of a longstanding collaboration in Photosystem II research between researchers at SLAC and Berkeley Lab.
Bergmann noted that there is still work to be done to sharpen the resolution of Photosystem II using X-ray lasers, which may be possible with improvements in the crystal samples and in the delivery systems used to stream the crystals across the X-ray laser beam.
There is an intense global scientific race to solve these long-kept secrets in photosynthesis, particularly with the launch of new capabilities in research made possible by X-ray lasers and the most advanced synchrotron sources.
Team co-leader Vittal K. Yachandra, a senior scientist in the Physical Biosciences Division at Berkeley Lab, said, “There is a lot of competition, but it is exciting because there is so much to do.”
Reference: “Room temperature femtosecond X-ray diffraction of photosystem II microcrystals” by Jan Kern, Roberto Alonso-Mori, Julia Hellmich, Rosalie Tran, Johan Hattne, Hartawan Laksmono, Carina Glöckner, Nathaniel Echols, Raymond G. Sierra, Jonas Sellberg, Benedikt Lassalle-Kaiser, Richard J. Gildea, Pieter Glatzel, Ralf W. Grosse-Kunstleve, Matthew J. Latimer, Trevor A. McQueen, Dörte DiFiore, Alan R. Fry, Marc Messerschmidt, Alan Miahnahri, Donald W. Schafer, M. Marvin Seibert, Dimosthenis Sokaras, Tsu-Chien Weng, Petrus H. Zwart, William E. White, Paul D. Adams, Michael J. Bogan, Sébastien Boutet, Garth J. Williams, Johannes Messinger, Nicholas K. Sauter, Athina Zouni, Uwe Bergmann, Junko Yano and Vittal K. Yachandra, 4 June 2012, Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.1204598109
Researchers from Stanford University Department of Chemistry, Technical University Berlin in Germany, European Synchrotron Radiation Facility in Grenoble, France and Umeå University in Sweden had key roles in this research and participated in the experiments at SLAC.







Be the first to comment on "Scientists Look to Photosynthesis for Cheaper and More Efficient Fuels"