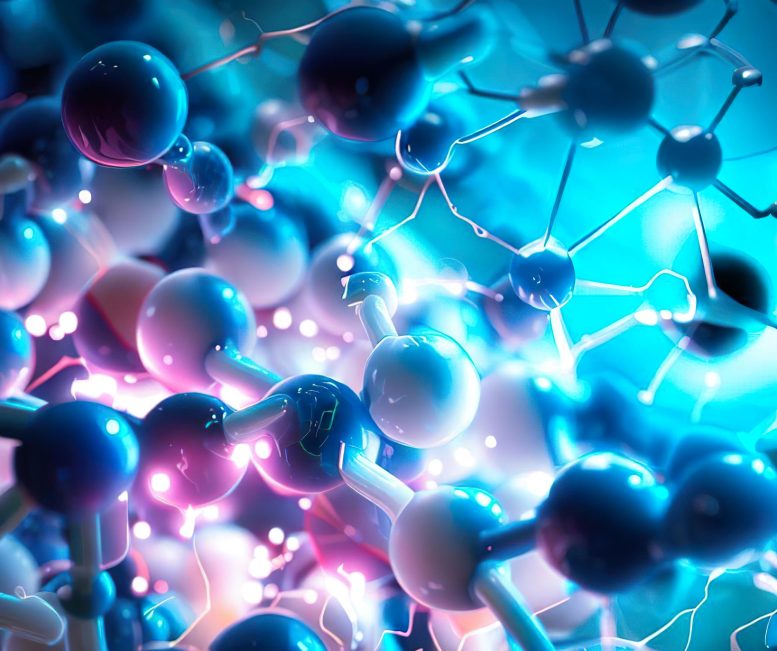
Artist’s illustration of an electronic polymer in water-conducting both ionic and electronic charges. Credit: Scott T. Keene
Researchers from the University of Cambridge have unveiled a surprising discovery that holds the potential to reshape the landscape of electrochemical devices. This new insight opens the door for the creation of cutting-edge materials and paves the way for enhancements in sectors like energy storage, neuromorphic computing, and bioelectronics.
Electrochemical devices rely on the movement of charged particles, both ions, and electrons, to function properly. However, understanding how these charged particles move together has presented a significant challenge, hindering progress in creating new materials for these devices.
In the rapidly evolving field of bioelectronics, soft conductive materials known as conjugated polymers are used for developing medical devices that can be used outside of traditional clinical settings. For example, this type of material can be used to make wearable sensors that monitor patients’ health remotely or implantable devices that actively treat disease.
The greatest benefit of using conjugated polymer electrodes for this kind of devices is their ability to seamlessly couple ions, responsible for electrical signals in the brain and body, with electrons, the carriers of electrical signals in electronic devices. This synergy improves the connection between the brain and medical devices, effectively translating between these two types of signals.
In this latest study on conjugated polymer electrodes, published in Nature Materials, researchers report on an unexpected discovery. It is conventionally believed that the movement of ions is the slowest part of the charging process because they are heavier than electrons. However, the study revealed that in conjugated polymer electrodes, the movement of “holes” – empty spaces for electrons to move into – can be the limiting factor in how quickly the material charges up.
Using a specialized microscope, researchers closely observed the charging process in real-time and found that when the level of charging is low, the movement of holes is inefficient, causing the charging process to slow down a lot more than anticipated. In other words, and contrary to standard knowledge, ions conduct faster than electrons in this particular material.
This unexpected finding provides valuable insight into the factors influencing charging speed. Excitingly, the research team also determined that by manipulating the microscopic structure of the material, it is possible to regulate how quickly the holes move during charging. This newfound control and ability to fine-tune the material’s structure could allow scientists to engineer conjugated polymers with improved performance, enabling faster and more efficient charging processes.
“Our findings challenge the conventional understanding of the charging process in electrochemical devices,” said first author Scott Keene, from Cambridge’s Cavendish Laboratory and the Electrical Engineering Division. “The movement of holes, which act as empty spaces for electrons to move into, can be surprisingly inefficient during low levels of charging, causing unexpected slowdowns.”
The implications of these findings are far-reaching, offering a promising avenue for future research and development in the field of electrochemical devices for applications such as bioelectronics, energy storage, and brain-like computing.
“This work addresses a long-standing problem in organic electronics by illuminating the elementary steps that take place during electrochemical doping of conjugated polymers and highlighting the role of the band structure of the polymer”, said George Malliaras, senior author of the study and Prince Philip Professor of Technology in the Department of Engineering’s Electrical Engineering Division.
“With a deeper understanding of the charging process, we can now explore new possibilities in the creation of cutting-edge medical devices that can seamlessly integrate with the human body, wearable technologies that provide real-time health monitoring, and new energy storage solutions with enhanced efficiency,” concluded Prof. Akshay Rao, co-senior author, also from Cambridge’s Cavendish Laboratory.
Reference: “Hole-limited electrochemical doping in conjugated polymers” by Scott T. Keene, Joonatan E. M. Laulainen, Raj Pandya, Maximilian Moser, Christoph Schnedermann, Paul A. Midgley, Iain McCulloch, Akshay Rao and George G. Malliaras, 6 July 2023, Nature Materials.
DOI: 10.1038/s41563-023-01601-5
The research was supported in part by the Engineering and Physical Sciences Research Council (EPSRC), part of UK Research and Innovation (UKRI), the European Union’s Horizon 2020 research and innovation program, the NVIDIA Academic Hardware Grant Program, Clare College, and the Royal Commission for the Exhibition of 1851. Scott Keene is a Marie Skłodowska-Curie Postdoctoral Fellow at the Cavendish Laboratory and the Department of Engineering’s Electrical Engineering Division.






Low dimensional spatiotemporal matter is the basic underlying structure of high-dimensional spatiotemporal matter. So far, relativity and quantum mechanics are the most successful theories for describing the essence of spacetime. If the so-called elementary particles and microscopic particles are high-dimensional spatiotemporal matter, rather than low-dimensional spatiotemporal matter, then using relativity and quantum mechanics to explain certain characteristics of elementary particles and microscopic particles may confuse people and potentially fall into the quagmire of pseudoscience.
In the microparticle world, there is no clear boundary between physics and chemistry.
What are the SHATTERING CONVENTIONAL WISDOM ?
The Principle of Thermodynamic photon – Quantum mechanical Oscillation in natural process can be used to describe ion- pair’s and electron pair’s motion in the electrochemistry,related to Bio-Physics.
Why emphasis on particle physics when electromagnetic waves rather than electrons are the true carrier of info