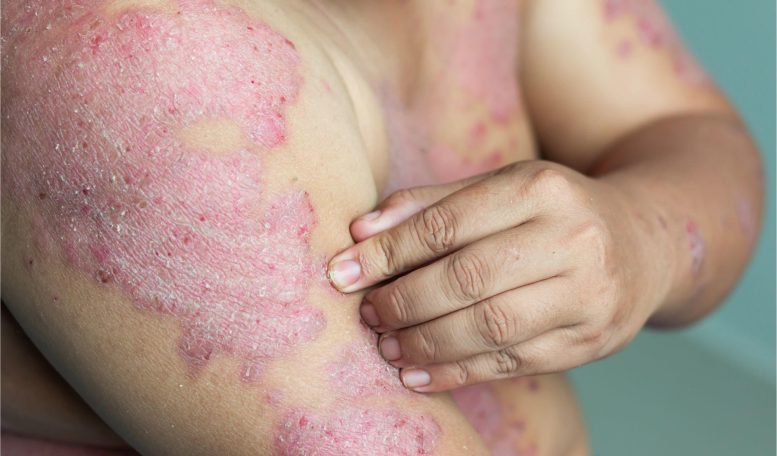
Eczema, also known as atopic dermatitis, is a chronic skin condition characterized by dry, itchy, and inflamed skin. It is a common condition that affects people of all ages, but it is most prevalent in infants and children. Eczema can cause the skin to become red, scaly, and crack, leading to discomfort, pain, and itchiness.
Patients experienced significant improvement in their symptoms, even up to 20 weeks after they had stopped taking the medication.
Mount Sinai researchers have reported promising results from a clinical trial of rocatinlimab, a new monoclonal antibody therapy that is tailored to individual patients, in the journal The Lancet. The trial was conducted on patients with moderate to severe atopic dermatitis, also known as eczema. The patients showed significant improvement in their symptoms while taking the drug, and the benefits persisted for up to 20 weeks after the therapy was discontinued.
According to the researchers, the results of the trial suggest that rocatinlimab has the potential to change the genetic makeup of a person’s eczema in the long term and potentially sustain lasting results even after the therapy is discontinued. The drug works by inhibiting OX40, an immune molecule that plays a key role in activating inflammatory cells, which contributes to the development of eczema and other inflammatory diseases.
“Atopic dermatitis, the most common type of eczema, is a debilitating chronic inflammatory skin disease that affects 1 in 10 Americans and millions of people worldwide,” said Emma Guttman, MD, Ph.D., Waldman Professor and System Chair, The Kimberly and Eric J. Waldman Department of Dermatology; Director, Center of Excellence in Eczema; and Director, Laboratory of Inflammatory Skin Diseases, at the Icahn School of Medicine at Mount Sinai. “It often develops at a very young age, causing the skin to become inflamed, red, extremely itchy, painful, and very dry—all symptoms that greatly affect a patient’s quality of life. We are very optimistic about the results of this trial and the potential for disease modification and long-lasting effects to improve patients’ quality of life.”
In this phase 2b multicenter, double-blind, placebo-controlled study, 274 patients were recruited and (rocatinlimab: n=217; placebo: n=57) randomly assigned 1:1:1:1:1 to rocatinlimab every four weeks (150 mg or 600 mg) or every two weeks (300 mg or 600 mg) or subcutaneous placebo up to week 18, with an 18-week active-treatment extension and 20-week follow-up. This trial was conducted at 65 sites within the United States, Canada, Japan, and Germany.
Percent change from baseline in the Eczema Area and Severity Index (EASI) score was assessed as the primary endpoint at week 16, and significance versus placebo was achieved with all active rocatinlimab doses (-48% to -61%) doses compared to placebo (-15%). All active dose cohorts also continued improving after week 16, and most patients maintained the response for at least 20 weeks off treatment.
The results support rocatinlimab as a safe and effective treatment for moderate to severe atopic dermatitis, with potentially long-lasting efficacy and disease modification. Adverse events reported were generally similar between rocatinlimab groups. Common adverse events during the double-blind period included fever, chills, headache, aphthous ulcers (canker sores), and nausea.
“At week 36, all participants had been on the treatment for at least 18 weeks,” added Dr. Guttman, senior author of the study. “By this time, we saw that while the drug achieved the primary endpoints in all doses versus the placebo, it’s also a drug that improves over time, which is really unusual and unique among currently available treatment options.”
Researchers plan to continue this investigation in a phase 3 program in 2023. Future studies will also include a larger study population, longer follow-up, and exploration of combination therapy (such as rocatinlimab plus topical corticosteroids).
Reference: “An anti-OX40 antibody to treat moderate-to-severe atopic dermatitis: a multicentre, double-blind, placebo-controlled phase 2b study” by Emma Guttman-Yassky, Eric L Simpson, Kristian Reich, Kenji Kabashima, Ken Igawa, Tetsuya Suzuki, Hirotaka Mano, Takeshi Matsui, Ehsanollah Esfandiari and Masutaka Furue, 9 December 2022, The Lancet.
DOI: 10.1016/S0140-6736(22)02037-2
The trial is registered on ClinicalTrials.gov (NCT03703102).





Be the first to comment on "Significant Improvements: A New, Promising Long-Term Treatment for Eczema"