Scientists identify a growth factor essential to the most common malignant pediatric brain tumor
In a newly published study, an international team of scientists has identified a molecular pathway that appears to be essential for the growth and spread of medulloblastoma, the most common malignant brain tumor in children.
In their report in the February 28 issue of the journal Cell, they show that blocking this pathway—which involves interactions between tumor cells and the surrounding tissues—leads to regression of all four molecular subtypes of medulloblastoma in several mouse models.
“Our finding that a pathway carrying signals from host cells to tumor cells via placental growth factor and its receptor neuropilin 1 is critical to the growth of medulloblastoma, regardless of molecular subtype, strongly supports evaluating antibodies against these proteins as a novel therapeutic approach to this pediatric cancer,” said Rakesh Jain, the A. Werk Cook Professor of Radiation Oncology (Tumor Biology) at Harvard Medical School, director of the Steele Laboratory for Tumor Biology at Massachusetts General Hospital and the study’s corresponding author.
A highly malignant tumor that originates in the cerebellum, medulloblastoma accounts for about 20 percent of all pediatric brain tumors and is 10 times more common in children than in adults. While aggressive treatment with surgery, chemotherapy, and radiation significantly improves patient survival, those treatments can have long-term developmental, behavioral, and neurological side effects, particularly in the youngest patients, making the need for less damaging therapies essential.
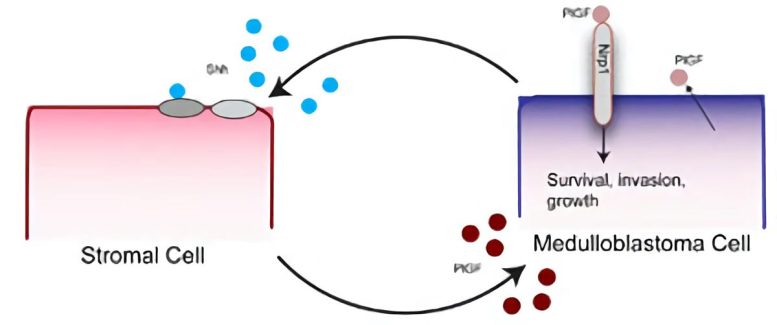
Medulloblastoma cells secrete the developmental protein Shh, which triggers nearby stromal, or connective, cells to produce placental growth factor (PlGF). Stromal-derived PlGF then binds to neuropilin 1 (Nrp1) receptors in the cancer cell and conveys signals that sustain the growth and spread of the tumor. Credit: Illustration courtesy of Lance Munn and Nathaniel Kirkpatrick of the Steele Laboratory for Tumor Biology, Massachusetts General Hospital.
Impetus for the current investigation began with studies by Peter Carmeliet of the Vesalius Research Center in Belgium, a co-author of the current study. Carmeliet found that an antibody against placental growth factor (PlGF) could block angiogenesis in a number of adult tumors. Because PlGF, unlike other angiogenic proteins, is not required for normal postnatal development, Jain and Lei Xu, HMS assistant professor of radiation oncology at Mass General, proposed targeting PlGF as anti-angiogenic treatment for pediatric tumors. Matija Snuderl, HMS clinical fellow in pathology at Mass General and a co-lead author of the current study, then found that PlGF was highly expressed in all types of medulloblastoma. Other members of Jain’s team found that high expression of the P1GF receptor neuropilin 1 (Nrp1) was associated with poor survival in medulloblastoma patients.
To investigate mechanisms behind the potential role of PlGF in medulloblastoma, the investigators collaborated with colleagues in the U.S., Belgium, Canada and Germany. They first confirmed that PlGF is expressed in patient samples of all subtypes of medulloblastoma and that expression of Nrp1 was more significant than that of PlGF’s more common receptor, VEGFR1. Experiments in several mouse models revealed that the presence of PlGF is essential for the progression of medulloblastoma and that treatment with several antibodies against the growth factor reduced tumor growth and spread, increasing animal survival even without substantially inhibiting angiogenesis.
The researchers were surprised to find that most PlGF was produced by surrounding supportive tissue called stroma and not by the tumor cells. Further investigation revealed that release of the developmental protein Shh (sonic hedgehog) by tumor cells induces expression in nearby stromal cells of PlGF, which then binds to the Nrp1 receptor on tumor cells, leading to further tumor growth. The authors noted that therapies that block the interaction between PlGF and Nrp1 are less likely to lead to treatment resistance than are therapies directly targeting mutations that drive tumor growth.
“The importance of tumor-stromal interactions has been recognized for decades, especially the formation of new blood vessels to supply tumors,” said Jain. “Our discovery of an entirely different way that tumor-stromal interactions drive cancer progression supports the exciting possibility that targeting that pathway in medulloblastoma could be more broadly effective with fewer side effects for patients. Antibodies against both PlGF and Nrp1 have been developed and tested in adult patients. There is hope that they could be safe in pediatric patients, but that needs to be established in clinical trials.”
Support for this study includes a grant from Hoffmann-La Roche and National Institutes of Health grant R01CA163815. Carmeliet has patent applications for intellectual property related to this study, and Jain is on the boards of trustees of H&Q Healthcare Investors and H&Q Life Science Investors.
Reference: “Targeting Placental Growth Factor/Neuropilin 1 Pathway Inhibits Growth and Spread of Medulloblastoma” by Matija Snuderl, Ana Batista, Nathaniel D. Kirkpatrick, Carmen Ruiz de Almodovar, Lars Riedemann, Elisa C. Walsh, Rachel Anolik, Yuhui Huang, John D. Martin, Walid Kamoun, Ellen Knevels, Thomas Schmidt, Christian T. Farrar, Benjamin J. Vakoc, Nishant Mohan, Euiheon Chung, Sylvie Roberge, Teresa Peterson, Carlos Bais, Boryana H. Zhelyazkova, Stephen Yip, Martin Hasselblatt, Claudia Rossig, Elisabeth Niemeyer, Napoleone Ferrara, Michael Klagsbrun, Dan G. Duda, Dai Fukumura, Lei Xu, Peter Carmeliet and Rakesh K. Jain, 28 February 2013, Cell.
DOI: 10.1016/j.cell.2013.01.036







Be the first to comment on "Study Finds a New Target in Childhood Brain Cancer"