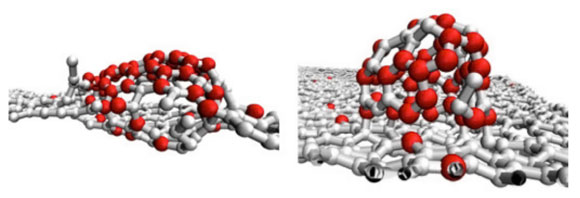
A computer simulation of the formation of complex organic molecules in space. The spherical molecular structures forming on the graphene surface at 3000 K are similar in shape to fullerenes. The red atoms originated in the gas phase and the white atoms are from the surface. Credit: Marshall and Sadeghpour
Scientists from the Harvard-Smithsonian Center for Astrophysics have used a new generation of supercomputers to simulate how atoms in space might combine to form carbon-rich molecules and clusters.
The space between stars is not empty, but contains an abundance of diffuse material, about 5-10% of the total mass of our galaxy (excluding dark matter). Most of the material is gas, predominantly hydrogen, but with a small and important component in complex carbon-bearing molecules including ethene, benzene, propynal, methanol and other alcohols, cyanides, simple amino acids, and even larger molecules (polycyclic aromatic hydrocarbons and buckyballs) with fifty or more carbon atoms. Some species like the cyanides have relative abundances similar to what is seen in comets in our Solar System, suggesting that the local carbon chemistry is not unique.
One important but unsolved issue for astronomers is how these complex organic molecules are made. The answer probably lies in the interstellar dust grains. These tiny grains are about one percent by mass of the interstellar material, and are made predominantly of silicates with some of carbon and/or other elements. These grains seem to be essential to the chemistry that takes place in the interstellar medium by providing gas molecules with a surface on which to react with other molecules.
CfA astrophysicists David Marshall and Hossein Sadeghpour have used a new generation of supercomputers (Harvard’s Odyssey research cluster) to simulate how atoms in space might combine to form carbon-rich molecules and clusters, either in the gas phase or on the surface of dust grains. Their simulations used temperatures ranging from 100 to 3000 kelvin (representative of values in stellar neighborhoods) and 4128 atoms (including both grain surface and gas phase atoms) – an unrealistically large number in context compared to the actual density of interstellar matter, but still very indicative of trends and the best computers can currently manage in reasonable calculation times, like days.
The scientists find that at low temperatures the grain surface helps to catalyze faster growth than happens in the gas phase, and that in general the surface temperature is a major factor in determining molecular structures, in particular their geometrical complexity. The research provides important new insights into the formation of large carbon-rich molecules on astrophysical carbonaceous surfaces. They conclude, for example, that although large chain and branched molecules can form on grain surfaces, they do not have enough energy to stick to the surface and so move around, whereas above 1000 kelvin they tend to stick to the surface and form isolated large clusters that might evolve into complex structures like buckyballs.
Reference: “Simulating the formation of carbon-rich molecules on an idealized graphitic surface” by David W. Marshall and H. R. Sadeghpour, 21 January 2016, MNRAS.
DOI: 10.1093/mnras/stv2524








Be the first to comment on "Supercomputers Simulate the Formation of Carbon-Rich Molecules in Space"