Groundbreaking research identifies a key function of neuron-OPC synapses in the brain, revealing their crucial role in myelin production. This discovery offers new insights into treating neurological conditions like MS, Alzheimer’s, and brain cancer. Credit: SciTechDaily.com
Discovery could be useful in developing new therapies for multiple sclerosis, neurodegenerative conditions, and brain cancer.
New research from Oregon Health & Science University for the first time reveals the function of a little-understood junction between cells in the brain that could have important treatment implications for conditions ranging from multiple sclerosis to Alzheimer’s disease, to a type of brain cancer known as glioma.
The study will be published today (January 12) in the journal Nature Neuroscience.
Neuroscientists focused on the junction, or synapse, connecting neurons to a non-neuronal cell, known as oligodendrocyte precursor cells, or OPCs. OPCs can differentiate into oligodendrocytes, which produce a sheath around nerves known as myelin. Myelin is the protective sheath covering each nerve cell’s axon — the threadlike portion of a cell that transmits electrical signals between cells.
The study found that these synapses play a pivotal role in producing that myelin.
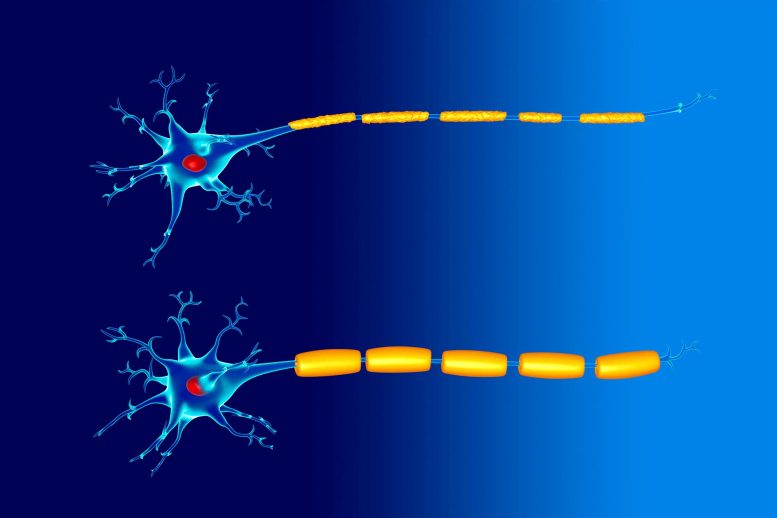
Illustration of two nerve cells (neurons). They both have a myelin sheath (yellow) around their axon, but the upper one is damaged.
“This is the first investigation of these synapses in live tissue,” said senior author Kelly Monk, Ph.D., professor and co-director of the Vollum Institute at OHSU. “This gives an understanding of the basic, fundamental properties of how these cells work in normal development. In the future, we might look at how they function differently in the context of MS patients.”
The fact that these synapses exist at all was the subject of a landmark discovery by OHSU researchers at the Vollum that was published in the journal Nature in May of 2000. Until that point, synapses in the brain had been known only to carry neurotransmitters between neurons, so the discovery of a synapse between neurons and OPCs came as a revelation.
“After two decades, we still didn’t know what these synapses do,” Monk said.
Scientists tackled the problem by using single-cell imaging of live tissue in zebrafish, whose transparent bodies enable researchers to see the inner workings of their central nervous system in real time. Using powerful new tools in imaging, pharmacology, and gene editing, researchers were able to use neuron-OPC synapses to predict the timing and location of the formation of myelin.
The findings are likely the tip of the iceberg in terms of understanding the importance of these synapses, said lead author Jiaxing Li, Ph.D., a postdoctoral fellow in Monk’s lab.
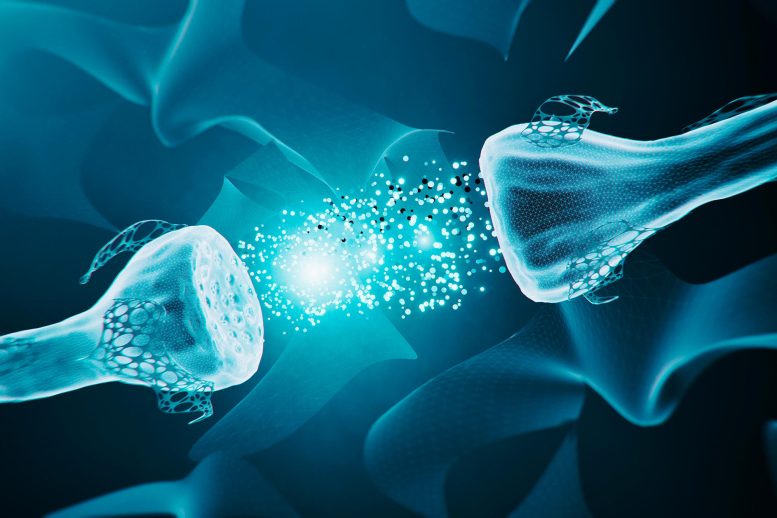
Neural synapses are the tiny gaps between neurons in the brain, serving as critical junctions where electrical or chemical signals are transmitted from one neuron to another, facilitating communication within the nervous system.
Oligodendrocyte precursor cells comprise about 5% of all cells in the brain — meaning the synapses they form with neurons could be relevant to many disease conditions, including the formation of cancerous tumors.
Li noted that previous studies have suggested a role for OPCs in a range of neurodegenerative conditions, including demyelinating disorders such as MS, neurodegenerative diseases such as Alzheimer’s, and even psychiatric disorders like schizophrenia.
By demonstrating the basic function of the synapse between neurons and OPCs, Li said the study may lead to new methods of regulating OPC function to alter disease progression. For example, these synapses could be the key to promoting remyelination in conditions such MS, where myelin has been degraded. In MS, this degradation can slow or block electric signals required for people to see, move their muscles, feel sensations, and think.
“There may be a way to intervene so that you can increase the myelin sheath,” he said.
Monk said the discovery may be most immediately relevant to cancer.
“In glioma, these synapses are hijacked to drive tumor progression,” she said. “It may be possible to modulate the synaptic input involved in tumor formation, while still allowing for normal synaptic signaling.”
Even though these precursor cells comprise roughly 5% of all human brain cells, only a fraction go on to form oligodendrocytes.
“It’s becoming pretty clear that these OPCs have other functions aside from forming oligodendrocytes,” Monk said. “From an evolutionary perspective, it doesn’t make sense to have so many of these precursor cells in your brain if they’re not doing something.”
Their synaptic connection to neurons therefore likely plays a fundamental role in the brain, and is worthy of future exploration, she said.
Reference: “Synaptic input and Ca2+ activity in zebrafish oligodendrocyte precursor cells contribute to myelin sheath formation” by Jiaxing Li, Tania G. Miramontes, Tim Czopka and Kelly R. Monk, 12 January 2024, Nature Neuroscience.
DOI: 10.1038/s41593-023-01553-8
In addition to Monk and Li, co-authors include Tania Miramontes of OHSU and Tim Czopka, Ph.D., of the Centre for Clinical Brain Sciences at the University of Edinburgh in the U.K.
The research was supported by the National Multiple Sclerosis Society postdoctoral fellowship, award FG-1907-34613 and the Warren Alpert Distinguished Scholar Award to Li; the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health, award number F31NS130898 to Miramontes; and NINDS award number 1R21NS120650 to Monk. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.







Be the first to comment on "The Brain’s Secret Handshake: Research Reveals Function of Little-Understood Synapse"