New research shows that uncontrolled growth in cancer cells leads to a state of senescence, affecting their ability to divide. This finding challenges current cancer treatment methods using growth and division inhibitors and suggests the need for alternative treatment strategies. Credit: SciTechDaily.com
ETH Zurich researchers are illuminating what can happen when cells exceed their normal size and become senescent. Their new findings could help to optimize cancer treatments.
- If cells in cell cultures grow while being treated with division-suppressing agents, their growth becomes excessive and they permanently lose their ability to divide.
- However, if the cells are treated with a combination of division inhibitors and growth inhibitors, they remain capable of dividing after these substances have been discontinued.
- The findings could be transferred to certain cancer therapies, but first need to be clinically tested and confirmed.
Understanding Cell Growth and Cancer
Growth is a fundamental biological process and a prerequisite for living organisms to develop and reproduce. The processes of cell growth (i.e. the production of new biomass) and of cell division must be coordinated with each other.
In multicellular organisms such as humans, the growth of cells must also be coordinated with their environment so that cells are present in the right number and size to form functional tissue or organs. Cell growth is therefore strictly regulated and takes place only when certain growth signals are present.
But cancer cells are different. They grow unchecked, they divide over and over again, and they don’t react to stop signals from their environment.
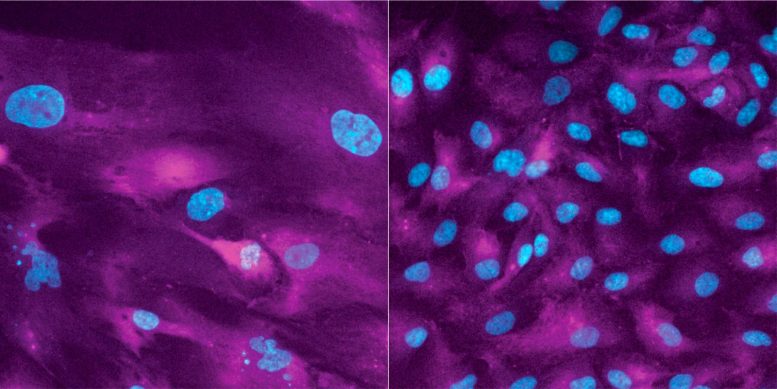
Cells in which only division is suppressed (left) continue to grow and lose their ability to divide, whereas cells in which growth and division are suppressed do not. Credit: Sandhya Manohar / ETH Zürich
A Dual Nature of Cancer Cells
Now several studies published in the journal Molecular Cell show that uncontrolled growth is not only an advantage for cancer cells but also a weakness.
One of these studies was led by Professor Gabriel Neurohr from the Institute of Biochemistry at ETH Zurich. For several years, he and his group have been researching how cell growth influences cell function. They are also investigating what happens when cells exceed their normal size and enter a state that the researchers refer to as senescence. In this state, the cells are preternaturally large and lose their ability to divide. Nevertheless, they are still active and can influence their environment, such as by releasing messenger substances.
Senescent cells are found in normal tissue and play an important role in the aging process. However, senescence can also be induced with chemical substances, and because it leads to a loss of the capacity to divide, it is the goal of certain cancer treatments.
A Breakdown in DNA Repair
Neurohr’s colleague Sandhya Manohar has now investigated whether excessive size affects cellular functions in senescent cells. In her research, she treated a non-cancerous cell line and a breast cancer cell line with substances that inhibit growth and division.
When she used only division-suppressing substances in her cell cultures, the cells were indeed no longer able to divide, but they continued to grow and went into senescence. As a result, they permanently lost their ability to divide. This effect persisted even after Manohar had discontinued the division inhibitors.
An important reason for the loss of the ability to divide is that the enlarged cells can no longer repair damage to their genetic material, such as double-stranded DNA breaks. Such breaks always occur spontaneously when a cell duplicates its genetic material prior to cell division.
In addition, these cells cannot correctly activate a key signaling pathway (p53-p21), which is critical for a coordinated response to DNA breaks. As a result, the damage is not repaired efficiently enough. What this means for enlarged cells is that numerous irreparable DNA breaks accumulate during division – to the point where division is no longer possible.
Questioning Combination Therapy in Cancer Treatment
Yet when the researchers treated the cells with division-inhibiting and growth-inhibiting substances simultaneously, the cells were able to divide and multiply normally again after both substances were discontinued. “In cancer therapy, this is precisely what you don’t want,” Neurohr says.
Growth- and division-inhibiting agents are already being used in cancer treatment. “Based on our observations in cell cultures, we would expect an increased relapse rate when treating a tumor with division inhibitors and growth inhibitors at the same time. It would make more sense to first use a division inhibitor, then a drug that further damages the DNA of the cells and makes division completely impossible,” Neurohr explains.
Further Research and Clinical Implications
Thus far, the ETH researchers have tested their new findings only on cell cultures. With both growth and division strongly dependent on the cell environment, the team cannot transfer these results directly to a clinical setting. Trials with organoids or on tissue samples are thus needed first to better test the potential treatment. Clinical studies investigating various combinations of division inhibitors and other medications are also underway.
The idea put forth by the ETH researchers under Neurohr has support from studies by three other international research teams, also published in the same issue of Molecular Cell.
These studies show that cancer cells with hyperactive growth are sensitive to treatment with division inhibitors. As these substances are already being used to treat certain types of breast cancer, the new findings could have a long-term impact on cancer treatment.
Reference: “Genome homeostasis defects drive enlarged cells into senescence” by Sandhya Manohar, Marianna E. Estrada, Federico Uliana, Karla Vuina, Patricia Moyano Alvarez, Robertus A.M. de Bruin and Gabriel E. Neurohr, 16 November 2023, Molecular Cell.
DOI: 10.1016/j.molcel.2023.10.018
This research was funded by an SNSF Professorial Fellowship for Gabriel Neurohr and an ETH Fellowship for Sandhya Manohar.







Be the first to comment on "The Paradox of Growth: Cancer’s Achilles’ Heel"