
A team of researchers has developed a biosynthetic genetic ‘clock’ that significantly extends cellular lifespan, as reported in the journal Science. The study involved genetically rewiring the gene regulatory circuit that controls cell aging, transforming it from a toggle switch to a clock-like device or gene oscillator. This oscillator periodically switches the cell between two detrimental aged states, thereby preventing prolonged commitment to either and slowing cell degeneration. The team used yeast cells in their study and achieved an 82% increase in lifespan compared to control cells. This ground-breaking research, underpinned by computational simulations and synthetic biology, could revolutionize scientific approaches to age delay, going beyond attempts to artificially revert cells to a state of ‘youth’. The team is now expanding its research to human cell types.
Studying yeast cells, researchers build a biosynthetic genetic ‘clock’ to extend lifespan.
Researchers have created a synthetic genetic ‘clock’ that significantly extends cellular lifespan. By reprogramming the gene regulatory circuit that controls aging, cells periodically switch between two detrimental states, slowing their degeneration. This innovative approach, tested on yeast cells, led to an 82% lifespan increase and could revolutionize age-delay strategies.
Human lifespan is related to the aging of our individual cells. Three years ago a group of University of California San Diego (UCSD) researchers deciphered essential mechanisms behind the aging process. After identifying two distinct directions that cells follow during aging, the researchers genetically manipulated these processes to extend the lifespan of cells.
As described on April 27, 2023, in the journal Science, they have now extended this research using synthetic biology to engineer a solution that keeps cells from reaching their normal levels of deterioration associated with aging. Cells, including those of yeast, plants, animals, and humans, all contain gene regulatory circuits that are responsible for many physiological functions, including aging.
“These gene circuits can operate like our home electric circuits that control devices like appliances and automobiles,” said Professor Nan Hao of the School of Biological Sciences’ Department of Molecular Biology, the senior author of the study and co-director of UC San Diego’s Synthetic Biology Institute.
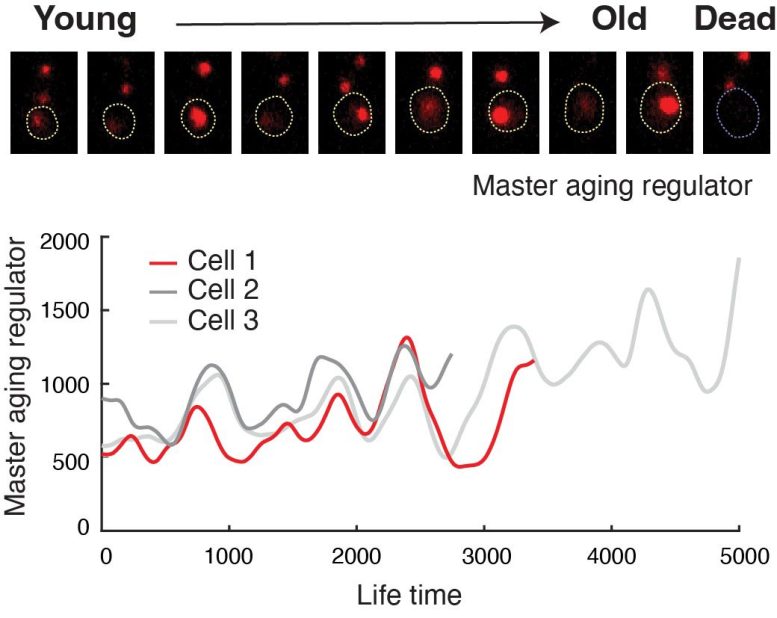
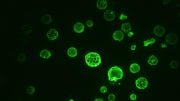
Engineered cells show oscillating abundance of a master aging regulator. Credit: Hao Lab, UC San Diego
However, the UC San Diego group uncovered that, under the control of a central gene regulatory circuit, cells don’t necessarily age the same way. Imagine a car that ages either as the engine deteriorates or as the transmission wears out, but not both at the same time. The UC San Diego team envisioned a “smart aging process” that extends cellular longevity by cycling deterioration from one aging mechanism to another.
In the new study, the researchers genetically rewired the circuit that controls cell aging. From its normal role functioning like a toggle switch, they engineered a negative feedback loop to stall the aging process. The rewired circuit operates as a clock-like device, called a gene oscillator, that drives the cell to periodically switch between two detrimental “aged” states, avoiding prolonged commitment to either, and thereby slowing the cell’s degeneration.
These advances resulted in a dramatically extended cellular lifespan, setting a new record for life extension through genetic and chemical interventions.
As electrical engineers often do, the researchers in this study first used computer simulations of how the core aging circuit operates. This helped them design and test ideas before building or modifying the circuit in the cell. This approach has advantages in saving time and resources to identify effective pro-longevity strategies, compared to more traditional genetic strategies.
“This is the first time computationally guided synthetic biology and engineering principles were used to rationally redesign gene circuits and reprogram the aging process to effectively promote longevity,” said Hao.
Several years ago the multidisciplinary UC San Diego research team began studying the mechanisms behind cell aging, a complex biological process that underlies human longevity and many diseases. They discovered that cells follow a cascade of molecular changes through their entire lifespan until they eventually degenerate and die. But they noticed that cells of the same genetic material and within the same environment can travel along distinct aging routes. About half of the cells age through a gradual decline in the stability of DNA, where genetic information is stored. The other half ages along a path tied to the decline of mitochondria, the energy production units of cells.
The new synthetic biology achievement has the potential to reconfigure scientific approaches to age delay. Distinct from numerous chemical and genetic attempts to force cells into artificial states of “youth,” the new research provides evidence that slowing the ticks of the aging clock is possible by actively preventing cells from committing to a pre-destined path of decline and death, and the clock-like gene oscillators could be a universal system to achieve that.
“Our results establish a connection between gene network architecture and cellular longevity that could lead to rationally-designed gene circuits that slow aging,” the researchers note in their study.
During their research, the team studied Saccharomyces cerevisiae yeast cells as a model for the aging of human cells. They developed and employed microfluidics and time-lapse microscopy to track the aging processes across the cell’s lifespan.
In the current study, yeast cells that were synthetically rewired and aged under the direction of the synthetic oscillator device resulted in an 82% increase in lifespan compared with control cells that aged under normal circumstances. The results revealed “the most pronounced lifespan extension in yeast that we have observed with genetic perturbations,” they noted.
“Our oscillator cells live longer than any of the longest-lived strains previously identified by unbiased genetic screens,” said Hao.
“Our work represents a proof-of-concept example, demonstrating the successful application of synthetic biology to reprogram the cellular aging process,” the authors wrote, “and may lay the foundation for designing synthetic gene circuits to effectively promote longevity in more complex organisms.”
The team is currently expanding its research to the aging of diverse human cell types, including stem cells and neurons.
Reference: “Engineering longevity—design of a synthetic gene oscillator to slow cellular aging” by Zhen Zhou, Yuting Liu, Yushen Feng, Stephen Klepin, Lev S. Tsimring, Lorraine Pillus, Jeff Hasty and Nan Hao, 27 April 2023, Science.
DOI: 10.1126/science.add7631
The research team, Zhen Zhou, Yuting Liu, Yushen Feng, Stephen Klepin, Lev Tsimring, Lorraine Pillus, Jeff Hasty and Nan Hao, are based across UC San Diego, including the Department of Molecular Biology (School of Biological Sciences), Synthetic Biology Institute, Moores Cancer Center (UC San Diego Health) and Shu Chien-Gene Lay Department of Bioengineering (Jacobs School of Engineering).




“Researchers at Northwestern University have extended the lifespan of fruit flies by 82% by genetically reprogramming their cells, reports SciTechDaily.” My God! Not fruit flies! This is as bad as Noah taking those two damn mosquitoes on the Ark. Who the heck wants fruit flies to live longer? I’m spending good money on trying to kill the pesky buggers.