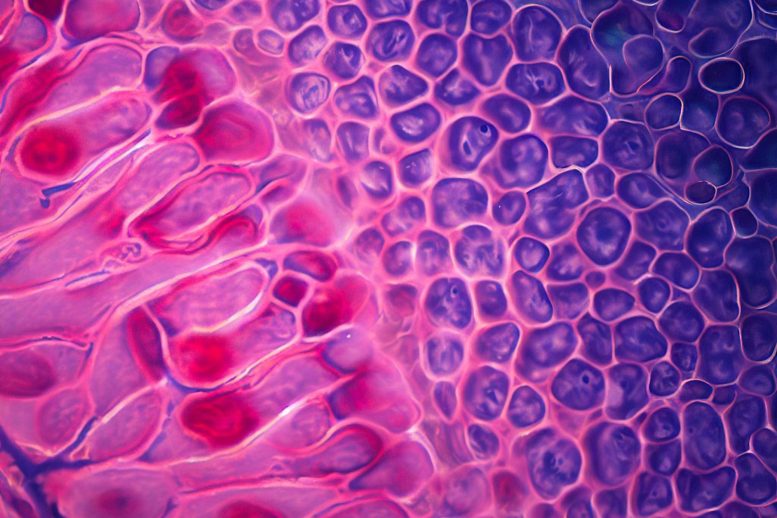
A groundbreaking study has mapped the molecular structures of integral components of the visual system, revealing how they degenerate over time and contribute to age-related macular degeneration. By sequencing the RNA of single cells, the researchers identified the gene ELN as a potential target for therapy against retinal diseases.
A landmark study published in the journal Genes & Diseases has substantially advanced our understanding of the human visual system.
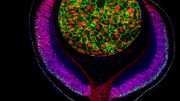
A groundbreaking study has taken a significant step toward understanding the complexities of the human eye. By mapping the molecular architecture of the retinal pigment epithelium (RPE) and choroid – integral components of the visual system – the research provides crucial insights into the cell compositions and molecular mechanisms underlying the eye’s changes with age and region.
The RPE and choroid, located behind the human retina, are fundamental to vision, playing a myriad of roles from light absorption to providing oxygenated blood to the photoreceptor cells. However, our understanding of the gene expressions within these cells and how they contribute to retinal diseases has been limited. Over time, the human RPE accumulates lipofuscin, an end product of phagosome breakdown, which weakens the RPE cells. Concurrently, the choroidal thickness decreases dramatically with age, reducing its blood flow. Both factors contribute to age-related macular degeneration (AMD), a condition that affects millions of people worldwide.
In a study published in the journal Genes & Diseases, researchers from Sichuan Provincial People’s Hospital revealed these degenerative processes and, importantly, identifies potential therapeutic targets.
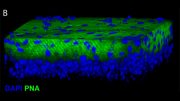
The study sequenced the RNA of approximately 0.3 million single cells from the human RPE and choroids across two regions at seven different ages. This detailed analysis has unveiled regional and age-specific differences between the human RPE and choroid. Such cellular interactions underscore the extensive connectivity networks between the RPE and different choroid cell types.
Additionally, the research team discovered that specific transcription factors and their target genes change during aging. Notably, they identified the gene ELN as a potential candidate for mitigating RPE degeneration and choroidal structure deterioration during aging, offering promising avenues for interventions in retinal diseases.
In conclusion, this study offers a comprehensive single-cell transcriptomic atlas of the human RPE and choroid across different regions and ages. It provides a wealth of information about the gene signatures of these integral components of the visual system. Moreover, the identification of ELN as a candidate for combating degeneration of choroidal and RPE structures paves the way for targeted interventions for anti-aging or ocular disease therapy.
This novel research has the potential to revolutionize our understanding of the human visual support system. It stands as a valuable resource for future studies into distinct gene-expression signatures and lays a solid foundation for future research into the functions of RPE and choroid genes.
Reference: “Dynamic human retinal pigment epithelium (RPE) and choroid architecture based on single-cell transcriptomic landscape analysis” by Lulin Huang, Lin Ye, Runze Li, Shanshan Zhang, Chao Qu, Shujin Li, Jie Li, Mu Yang, Biao Wu, Ran Chen, Guo Huang, Bo Gong, Zheng Li, Hongjie Yang, Man Yu, Yi Shi, Changguan Wang, Wei Chen and Zhenglin Yang, 15 December 2022, Genes & Diseases.
DOI: 10.1016/j.gendis.2022.11.007
Funding: National Natural Science Foundation of China, Sichuan Science and Technology Program, CAMS Innovation Fund for Medical Sciences




Be the first to comment on "Unraveling the Mysteries of Vision Degeneration: Pioneering Research Unveils Dynamic Human Eye Architecture"