Injecting calcium into graphene creates a superconductor, but where does the calcium actually end up? Credit: FLEET
‘Floating’ Graphene on a Bed of Calcium Atoms
Adding calcium to graphene creates an extremely-promising superconductor, but where does the calcium go?
Adding calcium to a composite graphene-substrate structure creates a high transition-temperature (Tc) superconductor.
Lead author Ph.D. student Jimmy Kotsakidis (School of Physics and Astronomy, Monash University). Credit: FLEET
In a new study, an Australian-led team has for the first time confirmed what actually happens to those calcium atoms: surprising everyone, the calcium goes underneath both the upper graphene sheet and a lower ‘buffer’ sheet, ‘floating’ the graphene on a bed of calcium atoms.
Superconducting calcium-injected graphene holds great promise for energy-efficient electronics and transparent electronics.
Studying Calcium-Doped Graphene: Throwing Off the Duvet
Graphene’s properties can be fine-tuned by injection of another material (a process known as ‘intercalation’) either underneath the graphene, or between two graphene sheets.
This injection of foreign atoms or molecules alters the electronic properties of the graphene by either increasing its conductance, decreasing interactions with the substrate, or both.
Injecting calcium into graphite creates a composite material (calcium-intercalated graphite, CaC6) with a relatively ‘high’ superconducting transition temperature (Tc). In this case, the calcium atoms ultimately reside between graphene sheets.
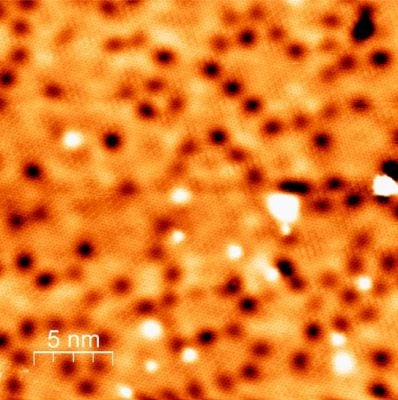
Measurements by STM (shown), XPS and LEED pinpointed the location of the calcium near the SiC surface. Credit: FLEET
Injecting calcium into graphene on a silicon-carbide substrate also creates a high-Tc superconductor, and we always thought we knew where the calcium went in this case too…
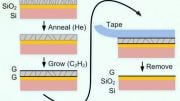
Graphene on silicon-carbide has two layers of carbon atoms: one graphene layer on top of another ‘buffer layer’: a carbon layer (graphene-like in structure) that forms between the graphene and the silicon-carbide substrate during synthesis, and is non-conducting due to being partially bonded to the substrate surface.
“Imagine the silicon carbide is like a mattress with a fitted sheet (the buffer layer bonded to it) and a flat sheet (the graphene),” explains lead author Jimmy Kotsakidis.
Conventional wisdom held that calcium should inject between the two carbon layers (between two sheets), similar to injection between the graphene layers in graphite. Surprisingly, the Monash University-led team found that when injected, the calcium atoms’ final destination location instead lies between buffer layer and the underlying silicon-carbide substrate (between the fitted sheet and the mattress!)

Research was carried out in the New Horizon labs, Monash University, as well as the Australian Synchrotron. Credit: FLEET
“It was quite a surprise to us when we realized that the calcium was bonding to the silicon surface of the substrate, it really went against what we thought would happen”, explains Kotsakidis.
Upon injection, the calcium breaks the bonds between the buffer layer and substrate surface, thus, causing the buffer layer to ‘float’ above the substrate, creating a new, quasi-freestanding bilayer graphene structure (Ca-QFSBLG).
This result was unanticipated, with extensive previous studies not considering calcium intercalation underneath the buffer layer. The study thus resolves long-standing confusion and controversy regarding the position of the intercalated calcium.
X-ray photoelectron spectroscopy (XPS) measurements at the Australian Synchrotron were able to pinpoint the location of the calcium near to the silicon carbide surface. Credit: FLEET
Reference: “Freestanding n-Doped Graphene via Intercalation of Calcium and Magnesium into the Buffer Layer–SiC(0001) Interface” by Jimmy C. Kotsakidis, Antonija Grubišić-Čabo, Yuefeng Yin, Anton Tadich, Rachael L. Myers-Ward, Matthew DeJarld, Shojan P. Pavunny, Marc Currie, Kevin M. Daniels, Chang Liu, Mark T. Edmonds, Nikhil V. Medhekar, D. Kurt Gaskill, Amadeo L. Vázquez de Parga and Michael S. Fuhrer, 15 July 2020, Chemistry of Materials.
DOI: 10.1021/acs.chemmater.0c01729
As well as the Australian Research Council (Laureate Fellowship and Centres of Excellence program), the authors acknowledge the support of the Australian Government Research Training Program, Monash Centre for Atomically Thin Materials (MCATM), Ministerio de Ciencia Innovación y Universidades, Comunidad de Madrid, and the US Naval Research Laboratory.
Computational support came from the Monash Campus Cluster, NCI computational facility and Pawsey Supercomputing Facility, and research was undertaken in part at ANSTO’s Australian Synchrotron.







This is outstanding information and the scientists who developed this matrix are to be awarded in some way. Personally our humanitarian organization is planning to build a very large number of 3 D copier manufactured houses that cannot be destroyed by earthquakes, forest fires or even plasma tourches, that ate totally waterproof, soundproof and will not support the growth of fungus or molds of any kind. I hope to meet with these scientists and fund many different applications, within a few months.
I hope someone runs an AI bot to search for papers like these and choose materials for developing new and improved structures that can help human beings to get over today’s main problems