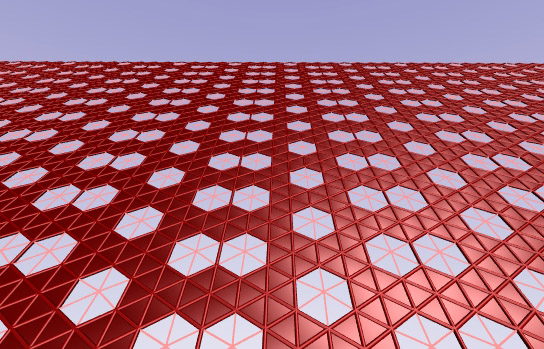
Rice University researchers calculated that two-dimensional sheets of purely metallic boron could take many forms, with clusters of vacancies where atoms drop out of the matrix, leaving hexagonal spaces. Credit: Evgeni Penev/Rice University
Scientists at Rice University used the notion of Swiss cheese to analyze the possible configurations of two-dimensional sheets of boron. They found that these 2-D sheets of boron, when rolled into tubes, could have a distinct advantage over carbon nanotubes since boron nanotubes are always metallic, avoiding the challenge of selecting a particular symmetry.
When is nothing really something? When it leads to a revelation about boron, an element with worlds of unexplored potential.
Theoretical physicist Boris Yakobson and his team at Rice University have taken an unusual approach to analyzing the possible configurations of two-dimensional sheets of boron, as reported this week in the American Chemical Society journal Nano Letters.
Treating it as Swiss cheese – in which the holes are as defining as the cheese itself – was the key concept in figuring out what atom-thin sheets of boron might look like. Those sheets, when rolled into a hollow tube, or nanotube, could have a distinct advantage over carbon nanotubes; boron nanotubes are always metallic, while the carbon atoms in a nanotubes can be arranged to form either metallic or semiconducting nanotubes. This variation in atomic arrangement — known as chirality — is one of the major hurdles to carbon nanotube processing and development.
“If I dream wildly, I like to think boron nanotubes would make a great energy-transporting quantum wire,” said Yakobson, Rice’s Karl F. Hasselmann Professor of Mechanical Engineering and Materials Science and professor of chemistry. “It would have the benefits of carbon, but without the challenge of selecting a particular symmetry.”
A boron lattice, even in just two dimensions, can have a range of configurations, Yakobson said. Fully packed, it’s a layer of atoms arranged in triangles. That’s one extreme. But take one atom out, and what was six triangles becomes a hexagon. Take all such possible atoms out and the sheet looks exactly like graphene, the two-dimensional, single-atom-thick form of carbon that has been all the rage in the world of chemistry and materials science for the past decade.
Between those two extremes are thousands of possible forms of pure boron in which missing atoms leave patterns of hexagonal holes.
“Carbon is well-defined,” said Yakobson, whose theories focus on the interactions at play among atoms as they bond and break. “Any deviation in graphene’s hexagonal form is what we call a defect, which has negative connotations.
“But we find there is a rich variety in two-dimensional boron,” he said. “It’s all purified – there’s no non-boron here, even though there are vacancies, empty sites. The amazing thing is that nature prefers to have it that way; Not hexagonal, where every third position is missing an atom, and not a triangular lattice. The optimum is right in the middle.”
In that most stable middle ground, the researchers found 10 to 15 percent of the boron atoms in a lattice were missing, leaving “vacancy concentrations” in a variety of patterns.
Yakobson said using traditional computational methods to assess thousands of boron configurations would have cost too much and taken too long. So he and Rice research scientist Evgeni Penev applied cluster expansion, a method of calculation more commonly applied to alloys.
“Evgeni gave it a twist: He treated the empty spaces as the second alloy ingredient, in the same way you can’t have Swiss cheese without ‘alloyed in’ voids and real cheese. In this calculation, the holes are an equal, physical entity.”
With space as a pseudoalloy, the researchers found a range of formation energies one might employ to identify stable sheets of boron with particular vacancy concentrations. They also found that synthesized boron layers would probably be polymorphic: Each sheet could contain a jumble of patterns and still be considered pure boron.
“Polymorphic means that all these possibilities are pretty much equal, and equally likely to form,” Yakobson said.
“This is a small part of the fundamental physics,” Penev said. “The next step is to consider more practical things, like whether it can be synthesized and under what conditions.”
Yakobson, who in 2007 first theorized the possibility of an 80-atom boron “buckyball,” said that while boron is difficult to work with, that difficulty makes it more rewarding. “On one hand, it’s very hard to conceive a possibility or to get experimental evidence. On the other hand, the field isn’t as crowded as graphene.”
Reference: “Polymorphism of Two-Dimensional Boron” by Evgeni S. Penev, Somnath Bhowmick, Arta Sadrzadeh and Boris I. Yakobson, 11 April 2012, Nano Letters.
DOI: 10.1021/nl3004754
Co-authors of the paper are Rice postdoctoral researchers Somnath Bhowmick and Arta Sadrzadeh.
The research was supported by the Department of Energy and the National Science Foundation through funding of Rice’s DAVinCI computer cluster.





Be the first to comment on "2-D Boron has Potential Advantages over Carbon Nanotubes"