Cancer is a disease characterized by the uncontrolled growth and spread of abnormal cells in the body. It is one of the leading causes of death worldwide, with various types of cancer affecting different parts of the body and having different symptoms. Despite advances in cancer research and treatment, the disease remains a significant public health concern.
Scripps Research scientists have identified how the structure of the PI3Kα protein changes in cancer cells, providing insight into potential drug-targeting strategies.
Understanding the structure of proteins that drive the growth of aggressive cancers is crucial in designing drugs that can effectively inhibit their growth.
Scripps Research Institute scientists have revealed the three-dimensional structure of phosphoinositide 3-kinase alpha (PI3Kα), a protein frequently mutated in cancer cells, in a series of three papers published in Proceedings of the National Academy of Sciences. Additionally, the research team has also provided insight into how this structure changes with cancer-associated mutations, which can open up new opportunities for drugs that can specifically target the mutated versions.
“We hope that these detailed structural findings lead to the discovery of drugs that affect cancer cells but not healthy cells,” says senior author Peter Vogt, Ph.D., a professor in the Department of Molecular Medicine at Scripps Research. “That could potentially eliminate the side effects associated with current PI3Kα drugs.”
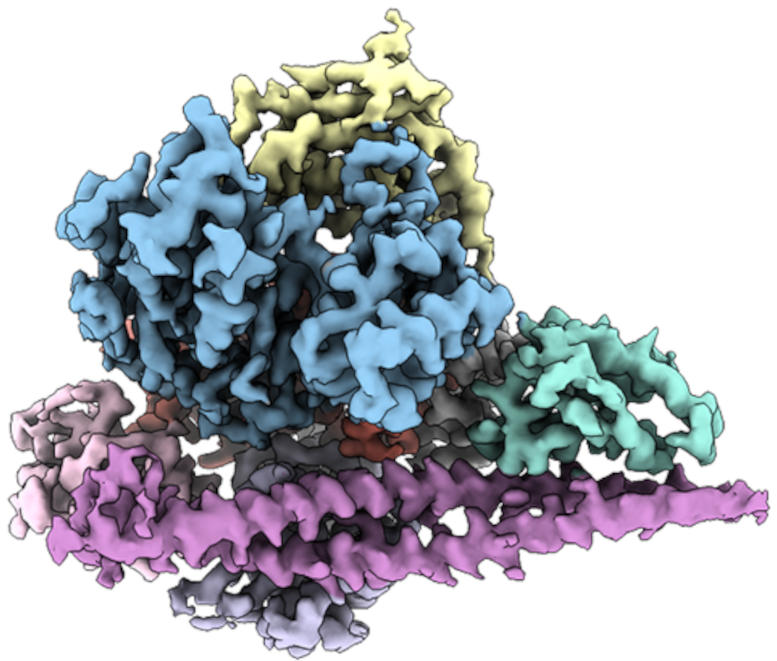
By determining the three-dimensional structure of PI3Kα (shown), Scripps researchers paved the way toward drugs that target the protein in cancer cells. Credit: Scripps Research
PI3Kα plays a central role in cell survival and growth. In healthy cells, the protein is flipped on and off as needed. But in numerous types of cancer—including breast, colorectal, endometrial, and brain—mutations in PI3Kα make it active all the time, encouraging the unchecked growth of the tumors. Current drugs that aim to put the brakes on PI3Kα bind to a section of the protein that rarely changes between healthy and mutated versions; this means all the PI3Kα in the body is shut off. Because of that, these PI3Kα inhibitors carry a long list of side effects and toxicities.
“To solve this problem, you have to make inhibitors that only recognize the mutated versions of PI3Kα,” says Vogt. “But to do that, you need structural information about what differentiates mutated, overactive PI3Kα from normal PI3Kα.”
This is no easy feat: PI3Kα is a particularly flexible, “wiggly” protein, so it’s difficult to get a single snapshot of its structure. Vogt’s group, however, discovered that when PI3Kα was bound to one of the existing inhibitors, it became more stable. In PNAS papers published in November 2021 and September 2022, they used a type of imaging technique known as cryogenic electron microscopy (cryo-EM) to work out the three-dimensional structure of PI3Kα. With this knowledge, they first examined the structure of PI3Kα attached to the inhibitor. Then, to visualize the protein without the inhibitor, they used cross-linking molecules to attach different parts of PI3Kα to itself, stabilizing the most flexible parts of the protein.
More recently, the research team used the same cryo-EM toolbox to piece together the structure of two mutated versions of PI3Kα often found in cancer cells. That work, published last month in PNAS, showed how some segments of the mutated PI3Kα resemble the activated form of PI3Kα.
“There are quite dramatic structural changes,” says Vogt. “And in the end, the changes essentially mimic the normal activated form of the protein, with the only difference being that it’s always in this active structure.”
The findings point toward ways to use drugs to shut off this always-on version of PI3Kα in cancer cells, without turning off healthy PI3Kα. The key, Vogt says, is that the drugs will need to bind to a different part of the PI3Kα protein than where the existing PI3Kα inhibitors bind—a part that varies structurally between the healthy and mutated versions of the protein.
His lab group is following up on this research with additional studies revealing how current drugs change the structure of PI3Kα.
References: “Cryo-EM structures of cancer-specific helical and kinase domain mutations of PI3Kα” by Xiao Liu, Qingtong Zhou, Jonathan R. Hart, Yingna Xu, Su Yang, Dehua Yang, Peter K. Vogt and Ming-Wei Wang, 7 November 2022, Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.2215621119
“Nanobodies and chemical cross-links advance the structural and functional analysis of PI3Kα” by Jonathan R. Hart, Xiao Liu, Chen Pan, Anyi Liang, Lynn Ueno, Yingna Xu, Alexandra Quezada, Xinyu Zou, Su Yang, Qingtong Zhou, Steve Schoonooghe, Gholamreza Hassanzadeh-Ghassabeh, Tian Xia, Wenqing Shui, Dehua Yang, Peter K. Vogt and Ming-Wei Wang, 12 September 2022, Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.2210769119
“Cryo-EM structures of PI3Kα reveal conformational changes during inhibition and activation” by Xiao Liu, Su Yang, Jonathan R. Hart, Yingna Xu, Xinyu Zou, Huibing Zhang, Qingtong Zhou, Tian Xia, Yan Zhang, Dehua Yang, Ming-Wei Wang and Peter K. Vogt, 1 November 2021, Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.2109327118
The research was funded by the National Cancer Institute.




Be the first to comment on "A How-To Guide to Designing Cancer Drugs"