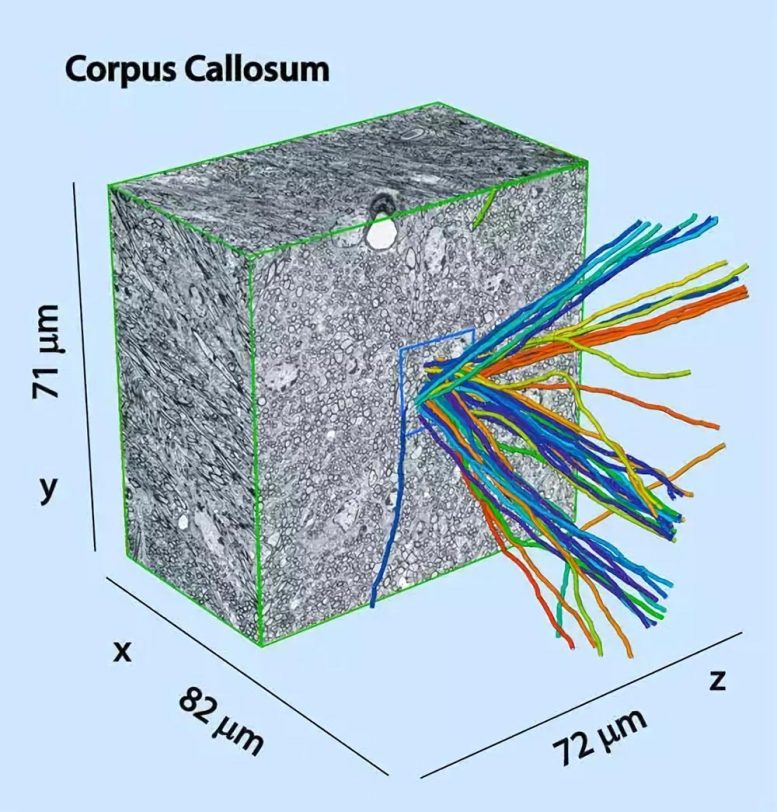
Serial block-face electron microscopy stack from the corpus callosum, cut down the middle, with 50 traced myelinated axons emerging, randomly colored. Credit: MPI for Medical Research
A team of Scientists at the Max Planck Institute for Medical Research has developed a method for preparing the whole mouse brain for “block-face” electron microscopy, a crucial step towards obtaining a complete circuit diagram of the brain of the mouse.
What happens in the brain when we see, hear, think and remember? To be able to answer questions like this, neuroscientists need information about how the millions of neurons in the brain are connected to each other. Scientists at the Max Planck Institute for Medical Research in Heidelberg have taken a crucial step towards obtaining a complete circuit diagram of the brain of the mouse, a key model organism for the neurosciences. The research group working with Winfried Denk has developed a method for preparing the whole mouse brain for a special microscopy process. With this, the resolution at which the brain tissue can be examined is so high that the fine extensions of almost every single neuron are visible.
Neurons transmit information through their extensions – the axons – and form a complex network of connections, which provides the basis for all information processing in the brain. Analyzing this network under the microscope is one of the biggest challenges facing the neurosciences. Most axons are less than one micrometer thick, some even smaller than 100 nanometers. “The electron microscope is the only microscope with a high enough resolution to enable individual axons lying next to each other to be distinguished from each other,” says Winfried Denk. Despite their minute diameter, axons can become very long and extend from one end of the brain to the other. To obtain an overall picture of a brain, the researchers have to analyze large pieces of tissue.
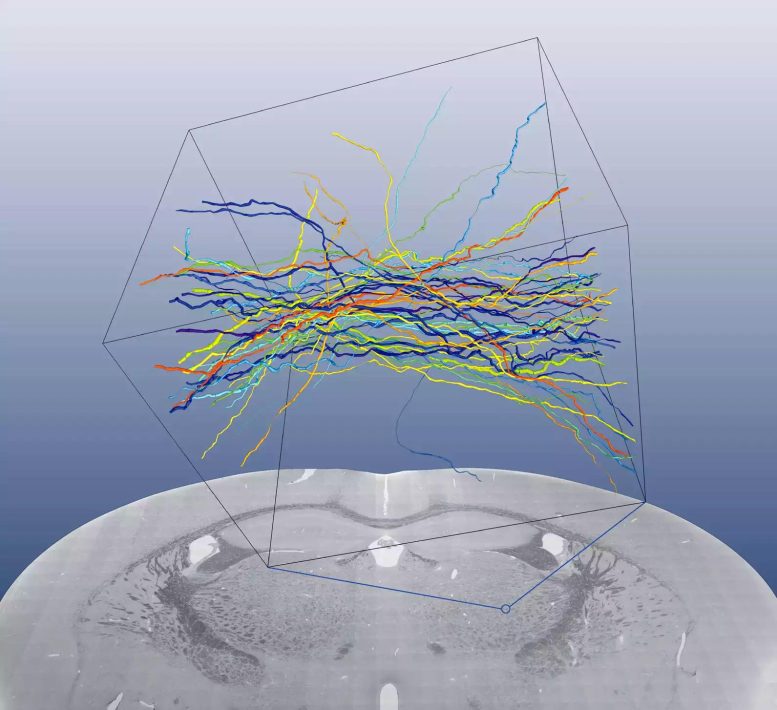
Scanning electron microscopic mosaic of a coronal blockface through a whole mouse brain acquired at 80 nanometer pixel size. Also shown are reconstructions of 50 myelinated axons from the ventroposterolateral nucleus (VPL) of the thalamus from a similarly-prepared sample. Each axon has a unique color. Credit: MPI f. Medical Research
In 2004, scientists working with Denk developed a new method that enabled them to do just this: “serial block-face” scanning electron microscopy. To examine tissue using this method, it must be fixed, stained and embedded in synthetic material. This works for small pieces of tissue, but up to now it was not possible for tissue the size of a mouse brain. In a current study, Shawn Mikula from Denk’s department succeeded in preparing a mouse brain in such a way that he was able to analyze it using block-face microscopy and trace the axons. The Max Planck research group would now like to image a whole brain with the “serial block-face” microscope so that they can study the neuronal connections in the entire mouse brain.
Reconstructed axons from the ventroposterolateral nucleus of the thalamus. Credit: Max Planck Institute
In their latest study, the Heidelberg-based researchers demonstrated that the brain of a mouse can be prepared in a way that enables it to be analyzed whole using “block-face” electron microscopy. The challenge facing the scientists was to treat a large piece of tissue so that it is evenly fixed and stained right through to the inside. To do this, they developed a complex process in which the brain is treated in different fixing and staining solutions for days.
With scanning electron microscopy, an electron beam scans the surface of a tissue section. A single electron microscope image thus corresponds to a cross-sectional view through the tissue. To obtain a three-dimensional image of a tissue, it is cut in fine sections using traditional methods, and these are then microscoped individually. This approach is not only tedious, it is also error-prone. Block-face microscopy overcomes this problem. This involves inserting an entire piece of tissue in the microscope and scanning the surface. Only then is a thin section cut, and the layer below is scanned. This makes it easier to combine the data on the computer.
Glimpse into the nerve tissue with block face electron microcospy: the procedure enables researchers to analyze the tissue layer by layer. The sequential layers depict a cross-section of cell extensions (circles with black borders) and cell bodies with the cell nucleus (grey circles). Credit: Max Planck Institute
In an initial analysis of the method, the scientists followed the axons of 50 randomly selected neurons and marked them by hand. The axons can be clearly reconstructed using the process. “However, it would take far too long to trace all of the neurons in this way as a mouse brain consists of around 75 million neurons,” says Denk. Therefore, the image evaluation must be automated. “Our images have sufficient resolution and contrast to follow all myelinated axons. If we manage to scan an entire brain in the years to come, this should provide a major incentive for computer scientists to develop the necessary analysis methods.”
A detailed map of the connections in the brain will make a major contribution to the clarification of neuronal functions. “Every theory on brain function is based on an idea of the corresponding information paths in the brain. It is very important that we find out about the connections between the nodes so that we can distinguish between different models of brain function,” explains Denk.
Reference: “A general method for chemogenetic control of peptide function” by Jiaqi Shen, Lequn Geng, Xingyu Li, Catherine Emery, Kayla Kroning, Gwendolyn Shingles, Kerry Lee, Matthias Heyden, Peng Li and Wenjing Wang, 8 December 2022, Nature Methods.
DOI: 10.1038/s41592-022-01697-8







This is abosolutely great and essential for neuroscience. Hurry up!
I have a question about the article, where can i speak to
the author?
Contact the Max Planck Institute.