
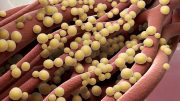
The cover art illustrates ultrasound-mediated drug delivery into a biofilm-infected wound. Credit: Ella Marushchenko
Scientists have developed a new strategy to improve the delivery of drugs to chronic wound infections.
Chronic wounds, which are persistent open sores or damaged tissues that do not heal correctly, pose a significant challenge to treat because of bacterial infections such as Staphylococcus aureus, or S. aureus. This situation is further complicated when these bacteria are resistant to antibiotics, such as methicillin-resistant S. aureus (MRSA), a primary source of severe infections in hospital environments.

Sarah Rowe-Conlon, Ph.D. Credit: UNC Department of Microbiology and Immunology
To protect itself from the human immune system and other potential threats, S. aureus can aggregate to form a slippery and slimy shield known as a biofilm. The biofilm barrier is so thick that neither immune cells nor antibiotics can penetrate through and neutralize the harmful bacteria.
Researchers at the UNC School of Medicine and the UNC-NC State Joint Department of Biomedical Engineering have developed a new method that combines palmitoleic acid, gentamicin, and non-invasive ultrasound to help improve drug delivery in chronic wounds that have been infected with S. aureus.
Using their new strategy, researchers were able to reduce the challenging MRSA infection in the wounds of diabetic mice by 94%. They were able to completely sterilize the wounds in several of the mice, and the rest had significantly reduced bacterial burden. Their results were published in Cell Chemical Biology.
“When bacteria are not completely cleared from chronic wounds, it puts the patient at high risk for the infection recurring or of developing a secondary infection,” said senior author Sarah Rowe-Conlon, Ph.D., a research associate professor in the Department of Microbiology and Immunology. “This therapeutic strategy has the potential to improve outcomes and reduce relapse of chronic wound infections in patients. We are excited about the potential of translating this to the clinic, and that’s what we’re exploring right now.”
Biofilms act as a physical barrier to many classes of antibiotics. Virginie Papadopoulou, Ph.D., a research assistant professor in the UNC-NCSU Joint Department of Biomedical Engineering, was curious to know if non-invasive cavitation-enhanced ultrasound could create enough agitation to form open spaces in the biofilm to facilitate drug delivery.

Paul Dayton, Ph.D. Credit: UNC-NCSU Joint Department of Biomedical Engineering
Liquid droplets which can be activated by ultrasound, called phase change contrast agents (PCCAs), are applied topically to the wound. An ultrasound transducer is focused on the wound and turned on, causing the liquid inside the droplets to expand and turn into microscopic gas-filled microbubbles, which then move rapidly.
The oscillation of these microbubbles agitates the biofilm, both mechanically disrupting it as well as increasing fluid flow. Ultimately, the combination of the biofilm disruption and the increased permeation of the drugs through the biofilm allowed the drugs to come in and kill the bacterial biofilm with very high efficiency.
“Microbubbles and phase change contrast agents act as local amplifiers of ultrasound energy, allowing us to precisely target wounds and areas of the body to achieve therapeutic outcomes not possible with standard ultrasound,” said Dayton, the William R. Kenan Jr. Distinguished Professor and Department Chair of the UNC-NCSU Joint Department of Biomedical Engineering. “We hope to be able to use similar technologies to locally delivery chemotherapeutics to stubborn tumors or drive new genetic material into damaged cells as well.”
When the bacterial cells are trapped inside the biofilm, they are left with little access to nutrients and oxygen. To conserve their resources and energy, they transition into a dormant or sleepy state. The bacteria, which are known as persister cells in this state, are extremely resistant to antibiotics.
Researchers chose gentamicin, a topical antibiotic typically ineffective against S. aureus due to widespread antibiotic resistance and poor activity against persister cells. The researchers also introduced a novel antibiotic adjuvant, palmitoleic acid, to their models.
Palmitoleic acid, an unsaturated fatty acid, is a natural product of the human body that has strong antibacterial properties. The fatty acid embeds itself into the membrane of bacterial cells, and the authors discovered that it facilitates the antibiotic’s successful entry into S. aureus cells and is able to kill persister cells and reverse antibiotic resistance.
Overall, the team is enthusiastic about the new topical, non-invasive approach because it may give scientists and doctors more tools to combat antibiotic resistance and lessen the serious adverse effects of taking oral antibiotics.
“Systemic antibiotics, such as oral or IV, work very well, but there’s often a large risk associated with them such as toxicity, wiping out gut microflora and C. difficile infection,” said Rowe-Conlon. “Using this system, we are able to make topical drugs work and they can be applied to the site of infection at very high concentrations, without the risks associated with systemic delivery.”
Reference: “Overcoming biological barriers to improve treatment of a Staphylococcus aureus wound infection” by Virginie Papadopoulou, Ashelyn E. Sidders, Kuan-Yi Lu, Amanda Z. Velez, Phillip G. Durham, Duyen T. Bui, Michelle Angeles-Solano, Paul A. Dayton and Sarah E. Rowe, 5 May 2023, Cell Chemical Biology.
DOI: 10.1016/j.chembiol.2023.04.009




Be the first to comment on "Breaking Bacterial Barriers: New Strategy Improves Treatment of Chronic Wound Infections"