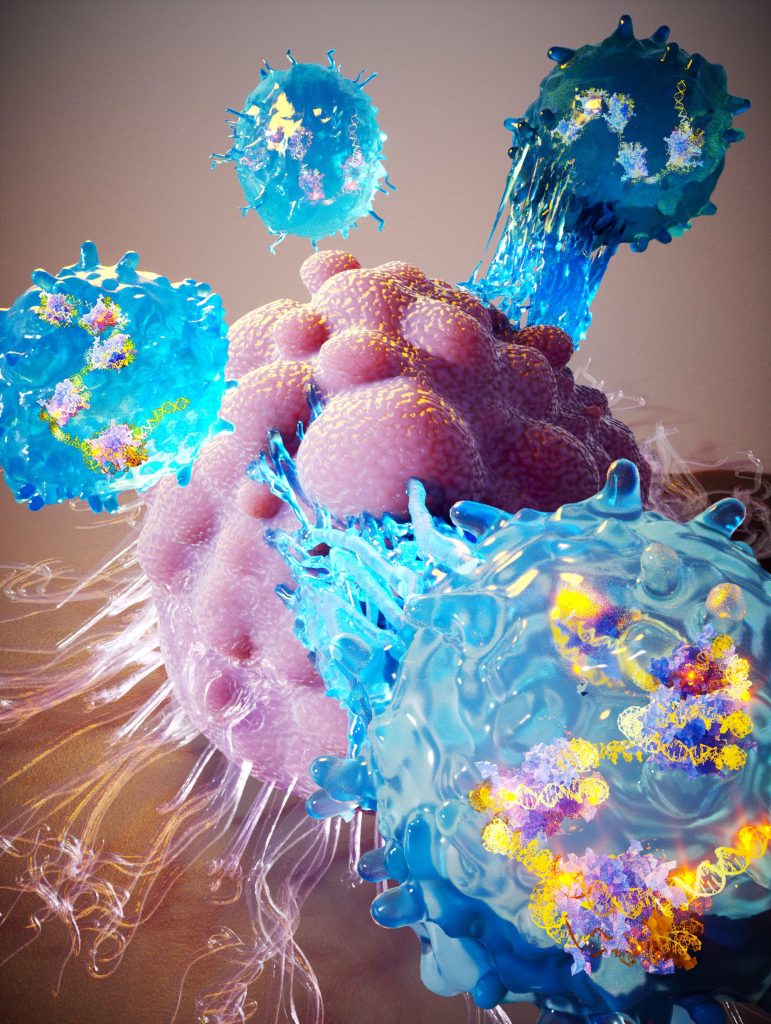
An artistic rendering of CRISPR-enhanced T-cells attacking a tumor. Research has shown that the gene-editing system CRISPR can modulate T-cell behavior to make them better cancer killers without actually editing any of their genes. Credit: Ella Maru Studio
A CRISPR-based platform has discovered numerous genes with the potential to enhance T-cell therapies for cancer treatment.
Researchers at Duke University have advanced CRISPR technologies for large-scale analysis of gene functions in human immune cells. They found that a single key regulator in the genome can reprogram a vast network of genes in T cells, significantly boosting their ability to kill cancer cells.
The master regulator is called BATF3 and is one of several genes that the researchers identified and tested for improving T-cell therapies. These targets, and the methods developed to identify, test, and manipulate them, could make any of the T-cell cancer therapies currently in use and under development more potent. Combined with other advances, the platform could also enable generalized, off-the-shelf versions of the therapy and expansion into other disease areas such as autoimmune disorders.
The results were recently published in the journal Nature Genetics.
Challenges in T-Cell Therapy and New Methodologies
T-cell therapy is a decade-old approach to treating cancer. More recent versions involve reprogramming the immune system’s primary soldiers to seek and destroy cancerous cells that they might otherwise overlook. Many companies are working to enhance the technology, mostly through the use of genetic engineering techniques that instruct the T cells on how to identify cancerous cells and make them more effective at destroying them.
There are currently six FDA-approved T-cell therapies for specific leukemias, lymphomas, and multiple myeloma. Their approaches, however, do not currently fare well when applied to solid tumors, although there are hints of success in certain studies. Solid tumors often present large physical barriers for the T cells to overcome, and the sheer number and density of cancer cells presenting targets can lead to “T-cell exhaustion,” wearing the attackers out to the point that they are not able to mount an antitumor response.
“In some cases, T-cell therapy works like a miracle drug, but in most others, it hardly works at all,” said Charles Gersbach, the John W. Strohbehn Distinguished Professor of Biomedical Engineering at Duke. “We are looking for generic solutions that can make these cells better across the board by reprogramming their gene regulation software, rather than rewriting or damaging their genetic hardware. This demonstration is a crucial step toward overcoming a major hurdle to getting T-cell therapy to work in more patients across a greater range of cancer types.”
Gersbach and his laboratory have spent the past several years developing a method that uses a version of the gene-editing technology CRISPR-Cas9 to explore and modulate genes without cutting them. Instead, it makes changes to the structures that package and store the DNA, affecting the activity level of the accompanying genes.
BATF3’s Role in Enhancing T-Cell Function
Sean McCutcheon, a Ph.D. candidate working in Gersbach’s lab and lead author of the study, focused on regions of this ‘dark genome’ that change as T cells transition between states, such as functional versus exhausted. He identified 120 genes that encode “master regulators,” which are responsible for the activity levels of many other genes. Using the CRISPR platform, he dialed the activity levels of these targets both up and down to see how they affected other known markers of T cell function.
While several promising candidates emerged, one of the most promising was a gene called BATF3. When McCutcheon subsequently delivered BATF3 directly to the T cells, there were thousands of tweaks to the packaging structure of the T cells’ DNA, and this correlated with increased potency and resistance to exhaustion.
“A known barrier to using T cells to fight cancer is that they tend to get ‘tired’ over time and lose their ability to kill cancer cells,” McCutcheon said. “We’re identifying manipulations that make T cells stronger and more resilient by mimicking naturally occurring cell states that work well in clinical products.”
The researchers put BATF3 through a battery of tests. The most interesting results came when they overexpressed BATF3 in T cells programmed to attack human breast cancer tumors in a mouse model. While the standard-of-care T-cell therapy struggled to slow tumor growth, the exact same dose of T-cells engineered with BATF3 completely eradicated the tumors.
Broad Implications and Future Research
While the results with BATF3 are exciting to Gersbach, McCutcheon, and the rest of the group, they are even more enthusiastic about the general success of the methodology to identify and modulate master regulators to improve therapeutic performance, which they have been developing for the better part of a decade. They can now readily profile master regulators of T cell fitness using any T cell source or cancer model and under various experimental conditions that mimic the clinical setting.
For example, in the last part of this study, McCutcheon screened T cells, with or without BATF3, while using CRISPR to remove every other master regulator of gene expression — more than 1,600 regulators in total. This led to the discovery of a whole new set of factors that could be targeted alone or in combination with BATF3 to increase the potency of T-cell therapy.
“This study focused in-depth on one particular target identified by these CRISPR screens, but now that Sean and the team have the whole discovery engine up and running, we can do this over and over again for different models and tumor types,” Gersbach said. “This study suggests many strategies for applying this approach to enhance T-cell therapy, from using a patient’s own T cells to having a bank of generalized T cells for a wide variety of cancers. We hope that these technologies can be generally applicable across all strategies.”
Reference: “Transcriptional and epigenetic regulators of human CD8+ T cell function identified through orthogonal CRISPR screens” by Sean R. McCutcheon, Adam M. Swartz, Michael C. Brown, Alejandro Barrera, Christian McRoberts Amador, Keith Siklenka, Lucas Humayun, Maria A. ter Weele, James M. Isaacs, Timothy E. Reddy, Andrew S. Allen, Smita K. Nair, Scott J. Antonia and Charles A. Gersbach, 9 November 2023, Nature Genetics.
DOI: 10.1038/s41588-023-01554-0
This research was supported by the National Institutes of Health (U01AI146356, UM1HG012053, UM1HG009428, RM1HG011123), the National Science Foundation (EFMA-1830957), the Paul G. Allen Frontiers, the Open Philanthropy Project, and the Duke-Coulter Translational Partnership.








Be the first to comment on "Harnessing the Dark Genome: New Approach Greatly Improves Cancer T-Cell Therapy"